To evaluate by optical coherence tomography neointimal healing response after implantation of cobalt-chromium-based titanium-nitride-oxide-coated (TiNO) stents and platinum-chromium-based biodegradable-polymer-coated everolimus-eluting stents (EES) in patients with acute coronary syndrome.
MethodsPatients were randomized (1:1) to receive either a TiNO-stent or EES. Optical coherence tomography images were obtained at 30-day (cohort A, n = 52) and 6-month (cohort B, n = 30) follow-up. The primary endpoint was the percentage of uncovered struts per patient.
ResultsIn cohort A, the percentage of uncovered struts was lower with TiNO-stents vs EES (3.2% vs 19.6%, P <.001). The percentage of malapposed struts was 0.4% in the TiNO-group and 2.1% in the EES group (P <.001). In cohort B, the percentage of uncovered struts was also lower with TiNO-stents (0.0% vs 8.7%, (P <.001). The percentage of malapposed struts was 0% in the TiNO-stent group and 0.3% in the EES group, (P=.008). None of the patients had repeat revascularization during the 6 months of follow-up.
ConclusionsFollowing percutaneous intervention for acute coronary syndrome, TiNO stent implantation was associated with a lower percentage of uncovered and malapposed struts per patient, compared with EES, at early and mid-term follow-up.
This study is registered under ClinicalTrials.gov, with number NCT02464397.
Keywords
Neointimal healing after stent implantation is a key determinant of long-term outcome. Concerns have focused on incomplete neointimal coverage of stent struts, which is the strongest risk factor for early and late stent thrombosis (ST).1–3 Optical coherence tomography (OCT) is the preferred invasive imaging modality for evaluating small degrees of neointimal coverage after stent implantation.4 The introduction of drug-eluting stents (DES) reduced repeat revascularization rates compared with bare-metal stents (BMS).5,6 Nevertheless, registry data showed higher rates of (very) late (ST) with first-generation DES compared with BMS.7 These safety concerns were addressed by the development of second-generation DES, and by prolonging the duration of dual antiplatelet therapy.8,9 In a recent meta-analysis, the incidence of late ST was lower with second-generation DES compared with first-generation DES and BMS.10 Consequently, recent guidelines recommend the use of second-generation DES over BMS.10 Titanium-nitride-oxide-coated (TiNO) stents have the theoretical advantage of more rapid and complete strut coverage compared with DES without the excessive intimal hyperplasia, as encountered with the BMS device.11–14 The safety of stainless-steel TiNO stents has been demonstrated in randomized clinical trials in patients with acute coronary syndrome (ACS) and recently in TIDES-ACS trial, which compared the same stents used in the present study.12,13 In previous OCT studies stainless-steel TiNO stents have shown better stent coverage compared with new-generation permanent-polymer everolimus-eluting stents (EES) in ACS patients.14–16 It is suggested that DES permanent polymers might be responsible for chronic inflammation, hypersensitivity reaction, delayed neointimal healing and neoatherosclerosis, which are known risk factors for (late) ST.17 Completely biodegradable-polymer DES were developed to overcome these limitations, although in clinical trials biodegradable-polymer DES have not shown a clear clinical benefit over permanent-polymer DES.18 We sought to evaluate by OCT the early and mid-term neointimal healing response following new-generation cobalt-chromium-based titanium-nitride-oxide-coated (TiNO)-stent (OPTIMAX) compared with platinum-chromium-based biodegradable-polymer EES (SYNERGY) implantation in patients presenting with ACS.
METHODSPatient selection and study designOPTIMAX-OCT is a prospective randomized study performed in 2 centers (Heart Center, Satakunta Central Hospital, Pori, Finland; Cardiovascular Center Aalst, Aalst, Belgium). The study compared neointimal healing response evaluated by OCT performed at 1- and 6-month follow-up of TiNO- stent (OPTIMAX, Hexacath, France) and EES (SYNERGY, Boston Scientific Corporation, United States) in patients presenting with ACS. The study enrolled patients with ACS and at least 1 significant (≥ 50% diameter stenosis) de novo lesion in a native coronary artery. Main exclusion criteria were cardiogenic shock, unprotected left main disease or ostial lesions, intolerance to study medications, and current participation in another study. Eligibility criteria are summarized in file 1 of the supplementary data. Eligible patients were randomly assigned in a 1:1 fashion to receive either a TiNO-stent or EES. Randomization was generated by computer-based software. Operators were by necessity not blinded to stent group allocation; however, the investigators who performed OCT analysis and data management, and patients were blinded. For follow-up purposes, patients were divided into cohort A, which underwent OCT evaluation at 30 days of follow-up, and cohort B, which underwent OCT evaluation at 6 months of follow-up. Patients underwent clinical follow-up at the assigned time point for the occurrence of major adverse cardiac events. The details of percutaneous coronary intervention, adjuvant pharmacological intervention, and the definitions of clinical endpoints are provided in file 2 of de supplementary data.
The study was initiated by the investigators and conducted according to the ethical guidelines of the 1964 Declaration of Helsinki, as revised in 2013. Informed written consent was obtained from every patient after full explanation of the study protocol. The protocol was reviewed and approved by the ethics committees of the participating centers. The OPTIMAX-OCT study is registered under ClinicalTrials.gov, with number NCT02464397.
Device descriptionThe OPTIMAX stent is a thin-strut (81μm) balloon-expandable stent, based on a cobalt chromium platform with a twin helicoidal design. The stent platform is coated with TiNO by plasma-enhanced vapor deposition of titanium in a prespecified gas mixture of nitrogen and oxygen, in a vacuum chamber. The SYNERGY stent is a thin-strut (74-81μm) balloon-expandable stent, based on a platinum-chromium platform coated with ultrathin (4μm) biodegradable Poly (D, L lactide-co-glycolide) abluminal polymer, which elutes everolimus (100μg/cm2).
Optical coherence tomography image acquisition and image analysisThe OCT image acquisition is presented in supplementary file 3. Proprietary software (St Jude Medical, St Paul, United States) was used to analyze cross-sections at 1-mm intervals (every 5 frames) within the stented segment. Stent cross-sectional area (CSA) and lumen CSA were traced semiautomatically. The neointimal hyperplasia (NIH) area was calculated by subtracting lumen CSA from stent CSA. The percent NIH area was calculated as the ratio of NIH area to the stent CSA, multiplied by 100. In each cross-section, the total number of analysable struts was counted. Struts were classified as uncovered if any part of the strut was visibly exposed to the lumen or covered if a layer of tissue was visible all over the reflecting surfaces. The percentage of uncovered struts was calculated as the ratio of uncovered to total struts multiplied by 100. In covered struts, NIH thickness was measured from the strut marker to the endoluminal edge of tissue coverage, following a straight line connecting the marker with the center of gravity of the vessel.19 Apposition was assessed by measuring the distance between the strut marker and lumen contour following a straight line connecting the marker with the center of gravity of the vessel. A margin of 18μm was added as a correction for half the blooming. Struts with distance to lumen contour greater than the sum of strut thickness (plus polymer thickness in case of EES) + 18μm were considered malapposed. Given a coated strut thickness of 81μm, we adopted a malapposition threshold of 100μm for TiNO-stents (81μm + 18=99μm). Similarly, given a strut thickness of 81μm and a polymer thickness of 4μm, we adopted a malapposition threshold of 100μm for EES (81μm + 4μm + 18=103μm). The percentage of malapposed struts was calculated as the ratio of malapposed to total struts multiplied by 100. Struts located at the ostium of a side branch were classified as nonapposed side branch struts and were excluded from the analysis. Thrombus was defined as an irregular high- or low-backscattering (red or white thrombus) mass protruding into the lumen discontinuous from the surface. Offline OCT analysis was performed independently by 2 investigators who were blinded to patient characteristics as well as the type of the stent used.
Statistical analysisSample size calculations were made based on previous OCT studies comparing TiNO-stents and new-generation permanent-polymer EES in ACS patients.14,15 We assumed that an average of 150 struts per patient would be analyzed. Sample size calculation was based on the percentage of covered struts per patient at 1 month (cohort A) and at 6 months (cohort B) of follow-up. The following assumptions were made:
At the 1-month follow-up, the mean percentage of covered struts per patient with TiNO- stents would be 96%, with EES 85%. A sample size of 50 patients (1:1; TiNO-stents vs EES, 25 vs 25) would be needed to reject the null hypothesis with a power of 90% (ß=0.90) and a 2-sided α of 0.05. The total sample size accounts for 5% loss to follow-up.
At the 6-month follow-up, the mean percentage of covered struts per patient with TiNO- stents would be 99%, with EES 92%. A sample size of 30 patients (1:1; TiNO-stents vs EES, 15 vs 15) would be needed to reject the null hypothesis with a power of 80% (ß=0.80) and a 2-sided α of 0.05. The total sample size accounts for 5% loss to follow-up.
Categorical variables are described as absolute and relative frequencies (percentage), whereas continuous variables are reported as median [interquartile range], or mean±standard deviation, as appropriate. The primary endpoint was the percentage of uncovered struts per patient, and the coprimary endpoint was the percentage of malapposed struts per patient, assessed at 30 days and 6 months of follow-up. To account for the nonindependence of struts in the same lesion, we adopted the method of nonparametric analysis of aggregated data for comparison of the percentage of uncovered (and malapposed) struts per patient between the 2 stent groups (patient-level analysis), in each cohort individually. In brief, the patient-level percentage was first calculated separately for each patient in a stent group, and then the median of these percentages was reported as the overall percentage estimator of the group.20 We also report the crude percentage of uncovered (and malapposed) struts for the whole stent group (strut-level analysis), in each cohort individually. The Pearson chi-square test, Fisher exact test, unpaired t test, or Mann-Whitney test were used for comparison of data between the 2 groups, as appropriate. All tests were 2-sided and a P value <.05 was considered statistically significant. Data were analyzed using SPSS v. 21 (SPSS IBM Inc, Unites States).
RESULTSBaseline characteristicsCohort AFrom January to October 2015, we enrolled 57 eligible patients: 30 patients received TiNO-coated stents, and 27 received EES. Two patients in the TiNO-stent arm and 3 in the EES arm withdrew consent, and did not attend follow-up. Finally, 52 patients were available for analysis (28 patients in the TiNO-stent arm and 24 in the EES arm). Mean age of cohort A was 62.9±9.0 years; 30.8% were females; 15.4% diabetic. Reference vessel diameter was smaller in patients who received EES; other baseline data were comparable (table 1). No major adverse cardiac events were observed in either stent arm at 30 days. No patients had ≥ 50% diameter stenosis at 30 days.
Baseline characteristics
| Cohort A | Cohort B | |||||
|---|---|---|---|---|---|---|
| TiNO-stent(n=28) | EES(n=24) | P | TiNO-stent(n=16) | EES(n=14) | P | |
| Age, y | 61.7±8.3 | 64.4±9.8 | .29 | 60.0±10.4 | 57.0±9.9 | .42 |
| Female sex | 8 (28.6) | 8 (33.3) | .71 | 4 (25.0) | 1 (7.1) | .20 |
| Hypertension | 14 (50) | 14 (58.3) | .54 | 12 (75.0) | 2 (14.3) | .001 |
| Hypercholesterolemia | 11 (39.3) | 12 (50) | .43 | 13 (81.3) | 4 (28.6) | .004 |
| Diabetes mellitus | 5 (17.9) | 3 (12.5) | .71 | 2 (12.5) | 0 (0.0) | .48 |
| Current smoking | 10 (35.7) | 7 (29.2) | .61 | 5 (31.3) | 7 (50.0) | .29 |
| Prior MI | 4 (14.8) | 2 (8.3) | .67 | 4 (25.0) | 2 (14.3) | .65 |
| Prior PCI | 5 (18.5) | 2 (8.3) | .42 | 2 (12.5) | 0 (0.0) | .48 |
| Prior CABG | 2 (7.4) | 0 (0) | .49 | 0 (0.0) | 0 (0.0) | NA |
| Presentation with ST-elevation MI | 8 (28.6) | 8 (33.3) | .71 | 1 (6.3) | 6 (42.9) | .06 |
| Index vessel | ||||||
| Left anterior descending | 12 (42.9) | 9 (37.5) | .90 | 8 (50.0) | 7 (50.0) | .19 |
| Left circumflex | 7 (25.0) | 6 (25.0) | 6 (37.5) | 2 (14.3) | ||
| Right coronary Artery | 9 (32.1) | 9 (37.5) | 2 (12.5) | 5 (35.7) | ||
| Calcified index lesion | 10 (35.7) | 6 (25.0) | .40 | 1 (6.3) | 3 (21.4) | .31 |
| Thrombus | 4 (14.3) | 4 (16.7) | 1.0 | 3 (18.8) | 4 (28.6) | .67 |
| Bifurcation | 2 (7.1) | 1 (4.2) | 1.0 | 0 (0.0) | 1 (7.1) | .46 |
| Reference vessel diameter (mm) | 3.15±0.29 | 2.83±0.55 | .02 | 2.87±0.49 | 3.12±0.35 | .12 |
| Diameter stenosis, % | 84±14 | 81±14 | .38 | 82±18 | 83±14 | .83 |
| Lesion length, mm | 14.6±4.6 | 14.5±4.2 | .92 | 16.0±5.5 | 18.3±2.9 | .18 |
| Preprocedural TIMI flow grade | 2.6±0.9 | 2.4±1.1 | .40 | 2.4±1.2 | 2.6±1.1 | .64 |
| Radial access | 24 (85.7) | 16 (66.7) | .10 | 15 (93.8) | 11 (78.6) | .31 |
| Predilation | 25 (96.2) | 20 (87.0) | .33 | 10 (71.4) | 11 (78.6) | 1.0 |
| Stent diameter | 3.21±0.30 | 3.14±0.35 | .38 | 3.08±0.37 | 3.30±0.44 | .15 |
| Stent length | 17.2±3.7 | 18.5±3.7 | .21 | 18.0±4.6 | 21.0±3.4 | .058 |
| Stent deployment pressure | 14.2±3.4 | 14.0±2.6 | .83 | 13.7±2.5 | 13.3±2.2 | .64 |
| No-reflow | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Postprocedural TIMI flow grade | 3.0±0.0 | 2.96±0.2 | .32 | 3.0±0.0 | 3.0±0.0 | NA |
| Stent failure | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Procedural success | 28 (100) | 24 (100) | NA | 16 (100) | 14 (100) | NA |
| Anti-thrombotic medication | ||||||
| Aspirin | 28 (100) | 24 (100) | NA | 16 (100) | 14 (100) | NA |
| P2Y12 inhibitor | 28 (100) | 24 (100) | NA | 16 (100) | 14 (100) | NA |
CABG, coronary artery bypass grafting; EES, everolimus-eluting stents; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis In Myocardial Infarction; TiNO, titanium-nitride-oxide.
Continuous variables are expressed as mean±standard deviation, whereas categorical variables are expressed as No. (%).
From December 2014 to April 2016, we enrolled 38 eligible patients: 20 patients received TiNO-coated stents and 18 received EES. Four patients in the TiNO-stent arm and 4 in the EES arm withdrew consent and did not attend follow-up. Finally, 30 patients were available for analysis (16 patients in theTiNO-stent arm and 14 in the EES arm). Mean age of cohort B was 58.6±10.1 years; 16.7% were female; 6.7% diabetic. Patients who received TiNO-stents were more often hypertensive and dyslipidemic; other baseline data were comparable (table 1). No major adverse cardiac events were observed in either stent arm at 6 months. No patients had ≥ 50% diameter stenosis at 6 months.
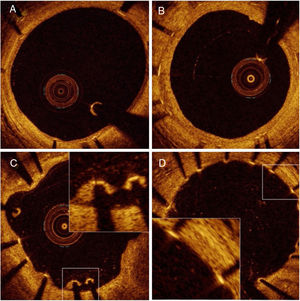
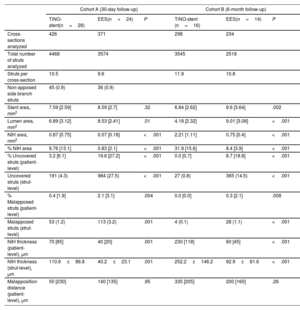
Optical coherence tomography dataCohort AOCT follow-up evaluation was performed at an average of 31.6±4.3 days in the TiNO-stent arm and 31.5±3.6 days in the EES arm (P=.97). OCT image acquisition was successful in all patients, and no OCT procedure-related complications were observed. We excluded from analysis 81 nonapposed side branch struts (45 in the TiNO-stent arm and 36 in the EES arm). We analyzed a total of 4468 struts in 426 cross-sections of TiNO-stent, and 3574 struts in 371 cross-sections of EES (table 2, figure 1). The percentage of uncovered struts per patient was lower in the TiNO-stent arm than in the EES arm (3.2% [6.1] and 19.6% [27.2], respectively, P <.001). Similarly, the percentage of malapposed struts per patient was lower in the TiNO-stent arm (0.4% [1.9] and 2.1% [3.1], respectively, P <.001). Nevertheless, NIH thickness was greater in the TiNO-stent arm, based on per-patient analysis (70 [85] and 40 [20] μm, respectively, P <.001), and per-strut analysis (110.6±86.8 and 40.2±23.1μm, respectively, P <.001). A small intrastent thrombus was observed by OCT in 2 patients in the TiNO-stent arm and in 7 patients in the EES arm.
Optical coherence tomography measurements
| Cohort A (30-day follow-up) | Cohort B (6-month follow-up) | |||||
|---|---|---|---|---|---|---|
| TiNO-stent(n=28) | EES(n=24) | P | TiNO-stent (n=16) | EES(n=14) | P | |
| Cross-sections analyzed | 426 | 371 | 298 | 234 | ||
| Total number of struts analyzed | 4468 | 3574 | 3545 | 2518 | ||
| Struts per cross-section | 10.5 | 9.6 | 11.9 | 10.8 | ||
| Non-apposed side branch struts | 45 (0.9) | 36 (0.9) | ||||
| Stent area, mm2 | 7.59 [2.59] | 8.59 [2.7] | .32 | 6.84 [2.62] | 9.6 [3.64] | .002 |
| Lumen area, mm2 | 6.89 [3.12] | 8.53 [2.41] | .01 | 4.18 [2.32] | 9.01 [3.08] | <.001 |
| NIH area, mm2 | 0.87 [0.75] | 0.07 [0.18] | <.001 | 2.21 [1.11] | 0.75 [0.4] | <.001 |
| % NIH area | 9.76 [13.1] | 0.83 [2.1] | <.001 | 31.9 [15.6] | 8.4 [3.9] | <.001 |
| % Uncovered struts (patient-level) | 3.2 [6.1] | 19.6 [27.2] | <.001 | 0.0 [0.7] | 8.7 [18.8] | <.001 |
| Uncovered struts (strut-level) | 191 (4.3) | 984 (27.5) | <.001 | 27 (0.8) | 365 (14.5) | <.001 |
| % Malapposed struts (patient-level) | 0.4 [1.9] | 2.1 [3.1] | .004 | 0.0 [0.0] | 0.3 [2.1] | .008 |
| Malapposed struts (strut-level) | 53 (1.2) | 113 (3.2) | .001 | 4 (0.1) | 28 (1.1) | <.001 |
| NIH thickness (patient-level), μm | 70 [85] | 40 [20] | .001 | 230 [118] | 60 [45] | <.001 |
| NIH thickness (strut-level), μm | 110.6±86.8 | 40.2±23.1 | .001 | 252.2±146.2 | 92.9±81.6 | <.001 |
| Malapposition distance (patient-level), μm | 50 [230] | 140 [135] | .95 | 335 [205] | 200 [160] | .28 |
EES, everolimus-eluting stents; NIH, neointimal hyperplasia; TiNO, titanium-nitride-oxide-coated.
Continuous variables are expressed as median [interquartile range] or mean±standard deviation, whereas categorical variables are expressed as No. (%).
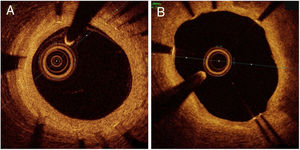
OCT follow-up evaluation was performed at an average of 185.9±22.4 days in the TiNO-stent arm vs 202.1±46.9 days in the EES arm (P=.28). Image acquisition was successful in all, and no complications were observed. We excluded from analysis 21 nonapposed side branch struts (all in the EES arm). We analyzed a total of 3545 struts in 298 cross-sections of TiNO-stent, and 2518 struts in 234 cross-sections of EES (table 2, figure 2). The percentage of uncovered struts per patient was lower in the TiNO-stent arm than in the EES arm (0.0 [0.7] and 8.7 [18.8], respectively, P <.001). Similarly, the percentage of malapposed struts per patient was lower in the TiNO-stent arm (0.0 [0.0] and 0.3 [2.1], respectively, P=.008). Nevertheless, NIH thickness was greater in the TiNO-stent arm, based on per-patient analysis (230 [118] and 60 [45] μm, respectively, P <.001), and per-strut analysis (252.2±146.2 and 92.9±81.6μm, respectively, P <.001). No thrombi were observed by OCT evaluation in either stent arm at 6-month follow-up.
DISCUSSIONMain findingsThe current study indicates that in patients who underwent early percutaneous coronary intervention for ACS, TiNO-stent implantation was associated with a greater extent of stent coverage, compared with EES, both at the 30-day and 6-month follow-up. In addition, the percentage of strut malapposition was smaller with TiNO-stent. However, NIH was more prominent after TiNO-stent than after EES implantation. To the best of the authorś knowledge, this is the first report of the OCT-evaluated comparative healing response of TiNO-stents and EES.
Neointimal healing and clinical perspectiveInadequate neointimal strut coverage is the most powerful predictor of early and late ST after stent implantation in histological autopsies and OCT report findings.3,21 Nevertheless, the percentage of uncovered and malapposed struts tends to decrease over time due to progressive neointimal growth and healing.22,23 Vascular healing is often delayed in patients treated with DES, especially in ACS patients.24 Stent strut coverage was adapted as a surrogate for stent safety. This is a clinically important question especially in patients with ACS patients when the risk of ST is higher in the early phase after PCI and with patients at high bleeding risk when shortened dual antiplatelet therapy is needed. Nearly one third of patients treated with PCI are considered to be at high bleeding risk.25 Few OCT studies reported early neointimal coverage after implantation of EES stent used in current study; EES showed higher portion of covered struts against permanent-polymer EES in early phase after stent implantation for ST-segment elevation myocardial infarction patients, uncovered struts were found in 57.6% at 2 weeks and in 28.4% at 4 months.26 In non–ST-elevation acute myocardial infarction patients uncovered struts occurred 21.5% at 1 month.27 Studies comparing previous-generation stainless-steel titanium-nitride-oxide-coated stents against permanent-polymer EES in ACS patients showed that uncovered struts occurred 1.2% at 2 months and 0.6% at 9 months with stainless-steel TiNO-stents compared with 11.3% and 10.8% with permanent-polymer EES.14,15 In one study with stainless-steel titanium-nitride-oxide stents (80% were ACS patients) the percentage of uncovered struts was 3.7% and NIH thickness was 71.5μm at 14 days after stent implantation.28 Furthermore, the NIH of titanium-nitride-oxide stents almost reached a plateau at 6 months, which is substantially earlier than the development of NIH with DES.29 After the healing process has finished (> 6 months), the NIH thickness of TiNO-stents falls between BMS and DES.14,16,30
There are no OCT studies comparing BMS to TiNO-stents. The results of the present study showing a low percentage of uncovered struts early after TiNO-stent implantation are in line with previous reports of neointimal healing of stainless-steel TiNO-stents.14,15,28 A recent randomized TIDES-ACS trial compared the stents used in the current study in ACS patients.13 TiNO-stents showed noninferiority to ESS for major cardiac events at 12 months and were superior to the coprimary safety endpoints of cardiac death, myocardial infarction and bleeding at 18 months with equal target lesion revascularization. There were more cardiac deaths, MI and ST at 12 months in the EES group, although the trial was underpowered to address these individual safety events. After the first year, the occurrence of cardiac death, MI and ST was low in both study groups.13 Interestingly, cardiac deaths, and MI between TiNO-stents and EES differed at an early phase after stent implantation,13 which was the period when strut coverage was lower with EES compared with TiNO-stents in the current study. This suggests that the differences in the early strut coverage and healing may at least partly explain these findings in early clinical events. The earlier and more adequate neointimal coverage of TiNO-stents comes at the expense of thicker NIH formation—which is expected—since DES are essentially designed to reduce in-stent restenosis. However, this did not result in excess target lesion revascularization in the randomized TIDES-ACS trial comparing TiNO-stents against ESS stents—the stents used in present study.13 In the current study no patients in TiNO-stent arm had ≥ 50% diameter stenosis and none underwent target lesion revascularization at 6 months. Since stent strut coverage is adopted as a surrogate for stent safety, the rapid coverage of TiNO-stents makes them safe to use in ACS patients.
LimitationsThe current study was based on a relatively small sample size; therefore, the results should be interpreted with caution. Moreover, the current OCT technology cannot detect neointimal coverage of less than 10-μm thickness and it is difficult to differentiate very thin layers of neointimal coverage between endothelium, thin layers of fibrin, or thrombus early after stenting (figure 1D). One limitation is that OCT was not performed before and immediately after the index procedure. Another clear limitation of the study is that 14% of the patients refused to participate in angiographic follow-up. Additionally, no independent core lab was involved in data analysis. Finally, the current study was underpowered to correlate OCT findings with clinical endpoints and larger studies are needed to address the clinical relevance of these findings.
CONCLUSIONSIn patients who underwent early percutaneous coronary intervention for ACS, TiNO-stent implantation was associated with a lower percentage of uncovered struts and malapposed struts per patient, compared with EES, as revealed by OCT performed at early and mid-term follow-up (figure 3). NIH thickness was greater in the TiNO-stent arm at both time points.
FUNDINGThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AUTHORS’ CONTRIBUTIONSJ. Sia confirms that all authors of this research article have made substantial contributions to the creation and design of the work, have directly participated in the planning and execution of the work, and have approved the final version submitted.
J. Sia and W. Nammas contributed equally.
CONFLICTS OF INTERESTThe authors have no conflicts of interest to declare.
- -
Inadequate neointimal strut coverage is the most powerful predictor of early and late stent thrombosis.
- -
The incidence of late ST is lower with second-generation DES than with first-generation DES and BMS.
- -
In a randomized clinical trial stainless-steel TiNO-stents have shown noninferiority to new-generation permanent-polymer ESS for major cardiac events.
- -
Early strut coverage was faster and more complete with TiNO-stents than with EES in patients with acute coronary syndrome.