New oral anticoagulants require dosing adjustment according to renal function. We aimed to determine discordance in hypothetical recommended dosing of these drugs using different estimated glomerular filtration rate equations in patients with atrial fibrillation.
MethodsCross-sectional analysis of 910 patients with atrial fibrillation and an indication for oral anticoagulation. The glomerular filtration rate was estimated using the Cockcroft-Gault, Modification of Diet in Renal Disease and Chronic Kidney Disease Epidemiology Collaboration equations. For dabigatran, rivaroxaban, and apixaban we identified dose discordance when there was disagreement in the recommended dose based on different equations.
ResultsAmong the overall population, relative to Cockcroft-Gault, discordance in dabigatran dosage was 11.4% for Modification of Diet in Renal Disease and 10% for Chronic Kidney Disease Epidemiology Collaboration, discordance in rivaroxaban dosage was 10% for Modification of Diet in Renal Disease and 8.5% for the Chronic Kidney Disease Epidemiology Collaboration. The lowest discordance was observed for apixaban: 1.4% for Modification of Diet in Renal Disease and 1.5% for the Chronic Kidney Disease Epidemiology Collaboration. In patients with Cockcroft-Gault<60mL/min or elderly patients, discordances in dabigatran and rivaroxaban dosages were higher, ranging from 13.2% to 30.4%. Discordance in apixaban dosage remained<5% in these patients.
ConclusionsDiscordance in new oral anticoagulation dosages using different equations is frequent, especially among elderly patients with renal impairment. This discordance was higher in dabigatran and rivaroxaban dosages than in apixaban dosages. Further studies are needed to clarify the clinical importance of these discordances and the optimal anticoagulant dosages depending on the use of different equations to estimate renal function.
Keywords
Over the last few years, there has been growing interest in the use of the new oral anticoagulants (NOAC). One direct thrombin inhibitor (dabigatran) and 2 factor Xa inhibitors (apixaban and rivaroxaban) have been tested in large phase III randomized trials, and they have all shown noninferiority or superiority in stroke prevention in patients with atrial fibrillation (AF) compared with adjusted-dose warfarin, with serious bleeding being similar to or less than that with monitored warfarin anticoagulation.1–3 This safety profile and the fact that these drugs do not require routine monitoring are important advantages given the wider indications for oral anticoagulation therapies in the current clinical guidelines for patients with AF4,5 and may explain their growing use in these patients.6 All of these NOACs are partially eliminated by renal clearance and require dosing adjustment according to renal function status; therefore, assessment of kidney function in patients with AF is of great importance.7
In 2010, the National Kidney Education Program recommended that the Modification of Diet in Renal Disease Study (MDRD) and Cockroft-Gault (CG) equations could be used interchangeably for drug dosing8 while the Kidney Disease: Improving Global Outcomes suggested that the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation may represent the method of choice for the staging of chronic kidney disease in this clinical setting.9 However, the European Medicines Agency seemed reluctant to recommend the use of these equations for this purpose because the phase III clinical trials evaluating NOAC in clinical practice have used only the CG equation for drug dose adjustment.10–12 Hence, the optimal glomerular filtration rate (GFR) estimation equations that should be used to assess an individual patient's GFR as a guide to the degree of adjustment of their drug dosage regimens remains controversial, especially among patients with moderate-severe renal impairment and/or the elderly.9
Many studies have compared multiple drug dosing recommendations based on different estimated GFR (eGFR) equations;13–18 but only a few have compared NOAC dosing recommendations based on the CG equation with those based on different eGFR equations.19,20 Moreover, none of these previous studies have compared the 3 NOACs currently incorporated into clinical practice guidelines. Consequently, the aim of the present study was to compare differences in hypothetical recommended dosing of dabigatran, rivaroxaban, and apixaban using kidney function estimates based on MDRD study and CKD-EPI equations in patients with AF.
METHODSStudy Population and DesignThe present study included consecutive patients with AF and indication for oral anticoagulation from our outpatient anticoagulation clinic. The patients’ complete medical history was recorded and informed consent was obtained from each patient at inclusion. The baseline stroke risk was assessed using the CHADS2 (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, and previous stroke or transient ischemic attack [doubled]) and CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled], vascular disease, age 65 to 74 years, and sex category [female]) scores, as described in recent guidelines. The HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly [> 65 years], drugs/alcohol concomitantly) bleeding risk score) was calculated as a measure of baseline bleeding risk. Patients on hemodialysis were excluded. The study was approved by the local ethics committees, and informed consent was obtained from each patient at inclusion.
Glomerular Filtration Rate EstimationEstimated GFR was calculated using the CG equation ([140 − age] × weight [kg]) / (sCr [mg/dL] × 72) (× 0.85 for women),21 the IDMS (Isotopic Dilution Mass Spectrophotometry)-traceable 4-variable MDRD study equation (175 × [sCr]–1.154 × [age]–0.203 [× 1.212 if African American] [× 0.742 if female]22 and the CKD-EPI equation: 141 × min (sCr / κ, 1)α × max (sCr / κ, 1)−1.209 × 0.993Age [× 1.018 if female] [× 1.159 if black], where“sCr” is serum creatinine; “κ” is 0.7 for female and 0.9 for male; “α” is –0.329 for female and –0.411 for male; “min” is the minimum of sCr / κ or 1, and “max” is the maximum of sCr / κ or 1.23 Data for the eGFR equations are expressed in millilitres per minute. Values for the MDRD and CKD-EPI equations (in mL/min/1.73 m2) were multiplied by each participant's body surface area and divided by 1.73 to yield units of millilitres per minute.
Statistical AnalysisContinuous variables were tested for normal distribution by the Kolmogorov-Smirnov test. Normally distributed data are presented as the mean (standard deviation) and non-normally distributed data as the median [interquartile range]. Categorical variables are expressed as percentages.
The variations of the MDRD and CKD-EPI equations were computed as the median [interquartile range] of the within-person difference between the value returned by each of the 2 equations and the CG equation. Agreement between the MDRD and CKD-EPI equations and CG equation was inspected visually by using Bland-Altman plots and quantified as the 95% limits of agreement between estimates. The limits of agreement represent a range of values within which the true difference between 2 methods can be said to lie with 95% confidence interval. For the 3 NOACs (Table 1), we identified dose discordance when there was disagreement in recommended dose based on the equations used to estimate GFR.24,25 For each NOAC, we quantified percentage dose discordance as 100 times the total number of participants with a dose discordance divided by the total number of participants studied. Cohen's kappa coefficient of agreement (κ) was used to detect the differences between the recommended doses for each pair of equations. All P values < .05 were accepted as statistically significant. Statistical analysis was performed using SPSS version 15.0 for Windows (SPSS, Inc.; Chicago, Illinois, United States).
New Oral Anticoagulants in Renal Dysfunction: Approved European Labels and Dosing in Chronic Kidney Disease
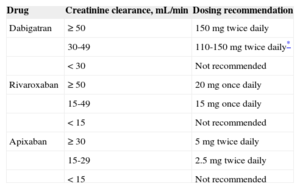
| Drug | Creatinine clearance, mL/min | Dosing recommendation |
|---|---|---|
| Dabigatran | ≥ 50 | 150mg twice daily |
| 30-49 | 110-150mg twice daily* | |
| < 30 | Not recommended | |
| Rivaroxaban | ≥ 50 | 20mg once daily |
| 15-49 | 15mg once daily | |
| < 15 | Not recommended | |
| Apixaban | ≥ 30 | 5mg twice daily |
| 15-29 | 2.5mg twice daily | |
| < 15 | Not recommended |
Where dabigatran is prescribed, a dose of 150mg twice daily should be considered for most patients in preference to 110mg twice daily, with the latter dose recommended in: elderly patients, age ≥ 80 years, concomitant use of interacting drugs (eg, verapamil), high bleeding risk (HAS-BLED score ≥ 3) or moderate renal impairment (CrCl 30–49mL/min). b.i.d denotes twice daily and once daily denotes once daily. Adapted with permission from Heidbuchel H et al.25
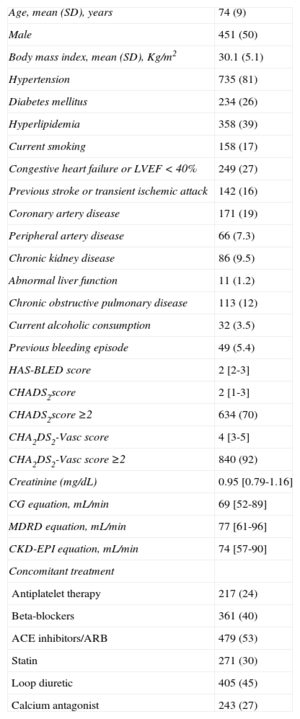
The study population consisted of 910 patients with AF. The clinical characteristics of study patients are summarized in Table 2. The median CHADS2 risk score was 2 [interquartile range, 1-3] and 634 (70%) participants had a CHADS2 risk score ≥ 2. The median CHA2DS2-VASc score was 4 [interquartile range, 3-5] and 840 (92%) participants had a CHA2DS2-VASc score ≥ 2. The estimated creatinine clearance obtained by using CG equation (69 [52-89] mL/min) was lower than those obtained using the MDRD and CKD-EPI equations (77 [61-96] mL/min and 74 [57-90] mL/min, respectively; both P < .001).
Clinical Characteristics of the Study Population (N = 910)
| Age, mean (SD), years | 74 (9) |
| Male | 451 (50) |
| Body mass index, mean (SD), Kg/m2 | 30.1 (5.1) |
| Hypertension | 735 (81) |
| Diabetes mellitus | 234 (26) |
| Hyperlipidemia | 358 (39) |
| Current smoking | 158 (17) |
| Congestive heart failure or LVEF < 40% | 249 (27) |
| Previous stroke or transient ischemic attack | 142 (16) |
| Coronary artery disease | 171 (19) |
| Peripheral artery disease | 66 (7.3) |
| Chronic kidney disease | 86 (9.5) |
| Abnormal liver function | 11 (1.2) |
| Chronic obstructive pulmonary disease | 113 (12) |
| Current alcoholic consumption | 32 (3.5) |
| Previous bleeding episode | 49 (5.4) |
| HAS-BLED score | 2 [2-3] |
| CHADS2score | 2 [1-3] |
| CHADS2score ≥2 | 634 (70) |
| CHA2DS2-Vasc score | 4 [3-5] |
| CHA2DS2-Vasc score ≥2 | 840 (92) |
| Creatinine (mg/dL) | 0.95 [0.79-1.16] |
| CG equation, mL/min | 69 [52-89] |
| MDRD equation, mL/min | 77 [61-96] |
| CKD-EPI equation, mL/min | 74 [57-90] |
| Concomitant treatment | |
| Antiplatelet therapy | 217 (24) |
| Beta-blockers | 361 (40) |
| ACE inhibitors/ARB | 479 (53) |
| Statin | 271 (30) |
| Loop diuretic | 405 (45) |
| Calcium antagonist | 243 (27) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CG, Cockcroft-Gault equation; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; MDRD, Modification of Diet in Renal Disease; SD, standard deviation; TIA, transient ischemic attack.
Data are expressed as mean (standard deviation), median [interquatile range] and No. (%).
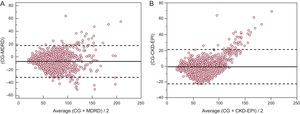
The mean within-participant differences relative to CG equation were –7.1mL/min [–8.0 to –6.3] for MDRD (P < .001) and –1.0mL/min [–1.7 to –0.2] for CKD-EPI (P < .001). The limits of agreement of MDRD and CKD-EPI equations with the CG equation were –32.0 to 17.7mL/min for MDRD and 3.2 to 21.2mL/min for CKD-EPI (Figure 1).
Bland and Altman plots showing the within-person differences between the estimated creatinine clearance obtained by using the Cockcroft-Gault equation and estimated glomerular filtration rate obtained by using (A) the Modification of Diet in Renal Disease equation and (B) the Chronic Kidney Disease Epidemiology Collaboration. The solid line indicates the mean difference, and the dashed line indicates limits of agreement. CG, Cockcroft-Gault; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease.
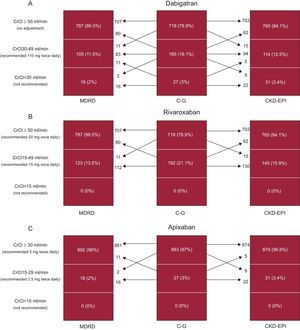
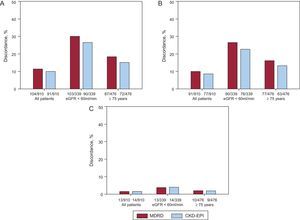
Figures 2A-C show the reclassification of patients and their recommended dosing for 3 NOACs according to renal function status using the 3 eGFR equations. The percentage discordance for the 3 NOACs was also calculated to quantify the implication of using the different methods for estimating kidney function on drug therapy (Figures 3A-C). Among the overall study population, the discordance in dabigatran dosage according to kidney function categories was 11.4% (n = 104) for MDRD (κ = 0.614; P < .001) and 10.0% (n = 91) for CKD-EPI (κ = 0.680; P<.001), whereas the discordance in rivaroxaban dosage relative to CG equation was 10.0% (n = 91) for MDRD (κ = 0.654; P<.001) and 8.5% (n = 77) for CKD-EPI (κ = 0.721; P < .001). The lowest discordance was observed for apixaban 1.4% (n = 13) for MDRD (κ = 0.704; P < .001) and 1.5% (n = 14) for CKD-EPI (κ = 0.751; P < .001).
Discordances in dosing of new oral anticoagulants as a function of estimated glomerular filtration rate equations. bid, twice daily; C-G, Cockcroft-Gault equation; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CrCl, creatinine clearance; MDRD, Modification of Diet in Renal Disease.
Discordance rates for the Modification of Diet in Renal Disease equation and chronic kidney disease epidemiology collaboration equations compared with recommended dosing based on the Cockcroft-Gault equation. A: dabigatran. B: rivaroxaban. C: apixaban. CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
In patients with moderate to severe renal impairment (CG<60mL/min, n = 339) the discordance was higher than in the whole study population. The discordance in dabigatran dosage was 30.4% (n = 103) for MDRD and 26.5% (n = 90) for CKD-EPI, whereas the discordance in rivaroxaban dosage relative to CG equation was 26.5% (n = 90) for MDRD and 22.4% (n = 76) for CKD-EPI. The lowest discordance was observed for apixaban, being 3.8% (n = 13) for MDRD and 4.1% (n = 14) for CKD-EPI.
In elderly patients (≥ 75 years, n = 476) the discordance was also higher than in the whole study population but lower than in patients with moderate to severe renal dysfunction. The discordance in dabigatran dosage was 18.3% (n = 87) for MDRD and 15.1% (n = 72) for CKD-EPI whereas the discordance in rivaroxaban dosage relative to CG equation was 16.2% (n = 77) for MDRD and 13.2% (n = 63) for CKD-EPI. In patients with renal function impairment, the lowest discordance was observed for apixaban, being 2.1% (n = 10) for MDRD and 1.9% (n = 9) for CKD-EPI (Figure 3C).
As detailed in Table 3, the use of MDRD or CKD-EPI equations was most likely to translate into higher recommended drug dosages, especially for dabigatran and rivaroxaban.
Concordance Among Drug Dosing Recommendations Using the Cockcroft-Gault Equation vs Estimated Glomerular Filtration Rate Obtained by Using the Modification of Diet in Renal Disease Equation and the Chronic Kidney Disease Epidemiology Collaboration
| MDRD | CKD-EPI | |||||
|---|---|---|---|---|---|---|
| Discordance | Discordance rate | Discordance | Discordance rate | |||
| < CG equation | > CG equation | < CG equation | > CG equation | |||
| Overall Study Population (N = 910) | ||||||
| Dabigatran | 104 (11.4) | 13 (12.5) | 91 (87.5) | 91 (10) | 24 (32.7) | 67 (67.3) |
| Rivaroxaban | 91 (10.0) | 11 (12.0) | 80 (88.0) | 77 (8.5) | 15 (19.5) | 62 (80.5) |
| Apixaban | 13 (1.4) | 2 (15.4) | 11 (84.6) | 14 (1.5) | 9 (64.3) | 5 (35.7) |
| Patients with CG equation < 60 mL/min (n = 339) | ||||||
| Dabigatran | 103 (30.4) | 12 (11.7) | 91 (88.3) | 90 (26.5) | 23 (25.6) | 67 (74.4) |
| Rivaroxaban | 90 (26.5) | 10 (11.1) | 80 (88.9) | 76 (22.4) | 14 (18.4) | 62 (81.6) |
| Apixaban | 13 (3.8) | 2 (15.4) | 11 (84.6) | 14 (4.1) | 9 (64.3) | 5 (35.7) |
| Patients ≥ 75 years (n = 476) | ||||||
| Dabigatran | 87 (18.3) | 3 (3.4) | 84 (96.6) | 72 (15.1) | 10 (13.9) | 62 (86.1) |
| Rivaroxaban | 77 (16.2) | 2 (2.6) | 75 (97.4) | 63 (13.2) | 5 (7.9) | 58 (92.1) |
| Apixaban | 10 (2.1) | 1 (10.0) | 9 (90.0) | 9 (1.9) | 5 (55.6) | 4 (44.4) |
CG, Cockcroft-Gault; CKD-EPI, chronic kidney disease epidemiology collaboration; MDRD, Modification of Diet in Renal Disease.
Data are expressed as No. (%).
The main findings of the present study can be summarized as follows: firstly, compared with the CG equation, both MDRD and CKD-EPI equations overestimate the eGFR. Secondly, when these equations are used instead of CG equation, the discordances were higher in dabigatran and rivaroxaban dosages than in apixaban dosages. Thirdly, most discordances were linked to an overestimation of renal function. Finally, among patients with CG<60mL/min and in elderly patients (≥75 years), the discordance in dabigatran and rivaroxaban dosages were higher than in the whole population, ranging from 13.2% to 30.4%. The discordance in apixaban dosage remained lower than 5% in this subgroup of patients.
The GFR is predominantly estimated in clinical practice from many estimating equations.26 Historically, the most frequently clinically used equation to estimate GFR has been the CG equation.21 This equation is reported in units not adjusted for body surface area, which is appropriate for drug dosage adjustment. The MDRD equation provides more accurate estimates of GFR than the CG equation18 and is now widely reported by clinical laboratories around the world whenever serum creatinine is reported.27 Nevertheless, scarce information has been published on the performance of this equation in elderly patients (such as those with AF requiring NOAC28) and it often overestimates measured GFR in patients with > 60mL/min/1.73 m2.27 The CKD-EPI equation was recently developed specifically to overcome this latter limitation, being more accurate than the MDRD study equation, particularly at higher levels of GFR.23,29 Although documentation of its utility for drug dosing is limited,30 it is likely to be similar to the MDRD equation, given the similar performance at lower levels of GFR, where dose adjustment is frequent.
Our study shows that the eGFR obtained with the MDRD and the CKD-EPI equations was consistently higher than that obtained with the CG equation. These findings agree with several retrospective studies in more than 20000 patients with chronic kidney disease, reporting that the use of the MDRD equation overestimates creatinine clearance, leading to significantly higher drug doses compared with doses calculated by using CG equation.13,14,31,32 The lack of appropriate renal dosage adjustments for NOACs may result in serious adverse events, and therefore our findings may have clinical significance.
As previously mentioned, when either the MDRD or CKD-EPI equations were used instead of the CG equation among patients with moderate to severe renal impairment, we found discordances in dabigatran and rivaroxaban doses of about 25%-30%. This finding is in agreement with a previous study showing a 50% discordance in dabigatran doses for MDRD or CKD-EPI equations in participants with GFR<30mL/min,33 with all cases resulting in higher doses being given compared with the use of the CG equation. Accordingly, we also found that most discordances were related to an overestimation of renal function in this subgroup of patients.
Strengths and LimitationsOne of the strengths of the present study is that it is the first to compare dabigatran, rivaroxaban, and apixaban dosing recommendations based on CG equation with those based on MDRD and CKD-EPI equations in an elderly cohort of patients with AF. According to our findings, a previous analysis of more than 4000 patients with AF in primary care showed that there would be clinically important potential risks when prescribing dabigatran or rivaroxaban if the MDRD formula was used instead of CG, especially in elderly patients. Among patients ≥ 80 years, 14.9% were ineligible for dabigatran according to the CG equation but would have been deemed eligible if the MDRD were applied. For rivaroxaban, 0.3% would have been incorrectly judged eligible for treatment and 13.5% would have received too high a dose.19 Moreover, Hijazi et al34 recently showed that rates of stroke, mortality, and major bleeding increase as renal function deteriorates. Both dabigatran doses (110mg and 150mg) displayed efficacy consistent with the overall trial relative to warfarin across the range of renal function in terms of the primary outcome of stroke or systemic embolism. By estimating GFR with the newer CKD-EPI equation, a significantly greater relative reduction in major bleeding risk was found for both doses of dabigatran in patients with eGFR > 80ml/min. In addition, an analysis of the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial revealed that a subset of participants >75 years without renal impairment had an increased risk of bleeding.35 Together with recent case reports of serious bleeding with dabigatran in older adults with decreased renal function,36–38 this finding further suggests that the higher doses of NOAC calculated using the MDRD and CKD-EPI equations could be potentially dangerous in older adults with reduced renal function. Interestingly, apixaban was the exception, as we found the lowest discordance between the 2 methods with this drug. A substudy of the ARISTOTLE trial39 on renal dysfunction showed that the percentage of patients with renal impairment was similar independently of the used method of assessment, although no comparisons were made. In this clinical trial setting, the apixaban dose was usually reduced by the presence of 2 of the following characteristics: age > 80 years, weight < 60kg or serum creatinine ≥ 1.5mg/mL3, and not by a reduction of eGFR as with dabigatran or rivaroxaban.1,2 However, as shown in Table 1, the recently published European Heart Rhythm Association practical guideline on the use of NOACs in AF patients recommends the use of apixaban (2.5 mg twice daily) in patients with an eGFR of 15-29mL/min.25
In 2010, the National Kidney Education Program and the Food and Drug Administration recommended that the MDRD and CG could be used interchangeably for calculating drug doses.8,40 In contrast to this recommendation, our results and those of several previous studies do not support the substitution of MDRD or CKD-EPI instead of the CG equation for calculating drug doses.32,41–44 In our opinion, clinicians need to fully understand the implications of using the MDRD and CKD-EPI equations instead of CG equation, particularly in patients with moderate to severe renal impairment, as well as in older persons, in whom the discordance among eGFR is highest among the 3 equations.33 It is important to note that neither Food and Drug Administration-approved drug labels nor the National Kidney Education Program recommendations are references for how to dose specific drugs in all patients. Differences between equations for estimating kidney function and drug dosing will always exist. Therefore, regardless of the equation used, clinical judgment must prevail. When presented with different kidney function estimates that potentially translate into different drug dosing regimens, clinicians must choose the regimen that optimizes the risk-benefit ratio given the patient-specific clinical scenario. When estimating equations are not expected to provide accurate measures of kidney function, it may be reasonable to obtain an accurately timed urine collection to calculate measured creatinine clearance.
The limitations of the present study include the small number of patients with severe renal dysfunction in the study population, which may have led to higher concordance than that expected in a population with a higher prevalence of severe chronic kidney disease. Moreover, we used drug dosage recommendations as an outcome, rather than actual observed drug dosage changes in clinical practice. Lastly, the lack of direct measures of GFR represents another important limitation of the study, but this method is rarely used in daily clinical practice.
CONCLUSIONSDiscordance in hypothetical recommended dosing of NOACs using different eGFR is frequent in patients with AF, especially among elderly patients with renal impairment. Remarkably, this discordance was higher in dabigatran and rivaroxaban dosages than in apixaban dosages. Further studies are needed to clarify the clinical importance of these discordances and the optimal anticoagulant dosages depending on the use of different GFR equations to estimate renal function in AF patients.
FUNDINGThis work was partially supported by PI 11/1256 from the ISCIII (Instituto de Salud Carlos III) and by IMIB (Instituto Murciano de Investigación Biosanitaria).
CONFLICTS OF INTERESTNone declared.