In heart transplantation (HTx), one of the long-term limitations is the development of neoplasia; the main causal factors of this are viral infections and immunosuppressive therapy.1 The proliferation signal inhibitors (PSIs) sirolimus and everolimus have been demonstrated to reduce the risk of cancer after transplant,2 although the immunosuppression protocols used were associated with an increase in graft rejection. It is not currently known which tumors will respond to the antineoplastic action of PSIs and therefore in which patients the benefits will outweigh the risks of rejection. One of the molecular pathways that is dysregulated in various tumor types is PI3K-AKT-mTOR, and this pathway is also one of the targets of PSIs.3
We compared the expression profile of several genes involved in the PI3K-AKT-mTOR pathway in tumor tissue and nontumor tissue from patients who had received a HTx, with the aim of identifying the genes with different expression that could be used as predictors of response to PSIs. We used samples of tumor and nontumor tissue from 16 patients who developed a neoplasm following HTx. These samples were taken from excess tissue obtained for clinical purposes, which was stored in the biobank of our hospital. The healthy tissue samples were taken from an unaffected area of the organ affected by the tumor. The RNA was acquired using a High Pure FFPET RNA Isolation Kit (Roche Diagnostics; Mannheim, Germany). DNA was obtained from the RNA via reverse transcription polymerase chain reaction using the RT2 First Strand Kit (Qiagen; Maryland, USA). After an initial analysis of samples from 3 patients, evaluating 84 genes involved in the PI3K-AKT-mTOR pathway, differential expression was detected in 34 genes. A systematic review of the available literature allowed us to conclude that only 27 had been demonstrated to be relevant, and these were therefore included in our final analysis. The variable analyzed was the mean value of DNA for each gene, adjusted for the constitutive gene HRPT1 (characterized by maintaining a constant level of expression in all the tissues of the same organism, according to the formula [mean value of tested gene–mean value of constitutive gene]/mean value of constitutive gene), expressed in tumor tissue compared with nontumor tissue. For this comparison, given the small sample size, the nonparametric Wilcoxon signed-rank test was used.
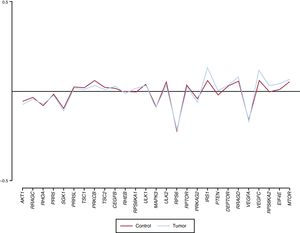
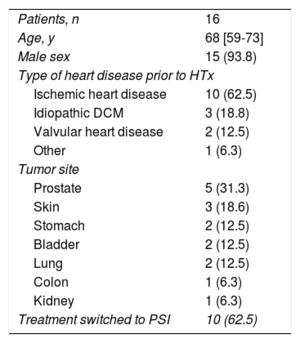
Sixteen patients were included in the study. The median age was 68 years [interquartile range, 59-73 years], and 93.8% were male. The most frequent reason for HTx was ischemic heart disease (62.5%) and the most prevalent tumor site was the prostate (31.3%). No patients were on treatment with a PSI before tumor diagnosis, but 10 patients (62.5%) were subsequently switched to receive one (Table). Only 1 gene (VEGFC) was observed to have an adjusted mean DNA value that was significantly higher in tumor tissue than in nontumor tissue (0.110 ± 0.066 vs 0.066 ± 0.087; P = .01). Two other genes had a tendency to increased expression in the tumor tissue, but this difference did not reach statistical significance (IRS1, P = .056; EIF4E, P = .063) (Figure).
Baseline Patient Characteristics
| Patients, n | 16 |
| Age, y | 68 [59-73] |
| Male sex | 15 (93.8) |
| Type of heart disease prior to HTx | |
| Ischemic heart disease | 10 (62.5) |
| Idiopathic DCM | 3 (18.8) |
| Valvular heart disease | 2 (12.5) |
| Other | 1 (6.3) |
| Tumor site | |
| Prostate | 5 (31.3) |
| Skin | 3 (18.6) |
| Stomach | 2 (12.5) |
| Bladder | 2 (12.5) |
| Lung | 2 (12.5) |
| Colon | 1 (6.3) |
| Kidney | 1 (6.3) |
| Treatment switched to PSI | 10 (62.5) |
DCM, dilated cardiomyopathy; HTx, heart transplant; PSI, proliferation signal inhibitor.
Unless otherwise indicated, data are expressed as median [interquartile grange] or no. (%).
This study, involving 16 patients who developed cancer after HTx, evaluated the hypothesis of a potential differential expression in tumor tissue but not in nontumor tissue of 27 genes involved in the PI3K-AKT-mTOR pathway. Only 1 of them (VEGFC) was statistically significantly overexpressed in the tumor tissue samples. The results of previous studies in nontransplant populations concur with this finding, and an increased expression of this gene has been found in various tumor types.4 The protein coded by this gene has a key role in angiogenesis, a process essential for tumor growth and metastasis.5 One of the mechanisms by which PSIs exert their antineoplastic effect is by reducing VEGFC expression and reducing endothelial cell sensitivity to this factor.6 The results of this study indicate that determination of the level of VEGFC gene expression in tumor tissue from patients who have developed cancer after HTx could help identify those with a potentially better response to the antineoplastic effects of PSIs, which would facilitate decision-making on the choice of immunosuppressive regimen to prescribe. More studies are needed to corroborate these findings.
FUNDINGThis study was performed with the help of a grant awarded by the Spanish Society of Cardiology in its 2013 annual call for basic research projects in cardiology. It was cofunded by the European Regional Development Fund (ERDF).
.
The authors thank Zulaika Grille-Cancela, Paula Blanco-Canosa, and Lucía Moreda-Santamaría for their help on this project.