We sought to compare the long-term clinical outcome of with ST-segment elevation myocardial infarction treated with paclitaxel-eluting stents or everolimus-eluting stents and the influence of thrombectomy on outcomes.
MethodsThe ESTROFA-IM is a multicenter retrospective registry collecting consecutive patients with infarction treated with these stents in 16 centers. Propensity-score matching was performed to select comparable stent groups and comparable groups with and without thrombectomy.
ResultsAfter matching patients, 350 treated with everolimus-eluting stents and 350 with paclitaxel-eluting stents were included in the analysis. The clinical and angiographic characteristics were comparable in both groups. The 2-year incidence of death, infarction, and target lesion revascularization was 14.9% for paclitaxel-eluting stents and 11.5% for everolimus-eluting stents (P = .04) and the incidence of definite/probable thrombosis 4.3% and 1.4%, respectively (P = .01). The use of paclitaxel-eluting was an independent predictor for events (hazard ratio = 2.44, 95% confidence interval, 1.28-4.65; P = .006). The benefit of everolimus-eluting stents over paclitaxel-eluting stents regarding stent thrombosis was more evident in the nonthrombectomy subgroup (5.4% vs 1.4%; P = .01). A significant interaction was found in the subgroups with and without thombectomy in the comparison between paclitaxel-eluting stents and everolimus-eluting stents for the end-point of stent thrombosis (P = .039).
ConclusionsThe results of this multicenter registry suggest better clinical outcomes with the everolimus-eluting stents in ST-segment elevation myocardial infarction. The lower risk of thrombosis with these stents could be more relevant in the absence of thrombectomy.
Keywords
Primary angioplasty in ST-segment elevation myocardial infarction (STEMI) represents the optimal reperfusion strategy.1 The safety of drug-eluting stents (DES) in this setting was initially questioned after the results of some large registries2–4 and a pathology study showing delayed vessel healing at the culprit site in STEMI patients treated with DES compared to patients receiving DES for stable angina.5
However, randomized trials and a large meta-analysis have shown that the use of DES is associated with a reduction in the need for new revascularization procedures, while the incidence of late thrombosis would not be increased with respect to bare metal stents.6–10 Yet, the available evidence is mainly coming from studies conducted with first-generation DES, particularly pacli-taxel-eluting stents (PES).8 A recently published meta-analysis has shown that the early benefit of early generation DES in primary angioplasty STEMI is offset in subsequent years by an increased risk of very late ST.11
Second-generation DES and mainly everolimus-eluting stents (EES) have demonstrated greater efficacy and safety than PES in both clinical trials and large registries.12–15 The EES have been compared to bare-metal stents and sirolimus-eluting stents in STEMI trials, suggesting a better profile for EES.16,17 Therefore it seems pertinent to evaluate EES in large STEMI registries and to compare them with PES.
On the other side, thrombectomy by means of aspiration has became a common practice in primary angioplasty when feasible, given the clinical benefit observed in clinical trials.18–21 However, after the publication of the INFUSE-AMI22 and TASTE23 trials the clinical efficacy of thrombectomy in STEMI remains uncertain.
Large thrombus burden has been identified as an independent predictor of stent thrombosis in patients treated with first-generation DES for STEMI.24 Therefore, the use or not of thrombus aspiration could have a differential effect on the clinical outcomes with different DES generations.
The main objective of this multicenter study was to compare the long-term clinical outcome of 2 large series of patients treated with PES or EES during routine clinical practice of primary angioplasty.
METHODSWe designed a multicenter retrospective registry involving 16 centers throughout Spain that volunteered their participation. It was officially supported by the Spanish Working Group of Interventional Cardiology of the Spanish Society of Cardiology and is part of the ESTROFA project and study network.
In every participating center, 2 consecutive series of patients that had undergone primary angioplasty procedures, with either PES (TaxusTM) or EES (Xience VTM or PromusTM), were included. In order to reduce selection bias, included patients treated with PES were those treated in the period just before the availability of EES in each center, between 2005 and 2007. The recruitment for patients treated with EES comprised the period between 2006 and 2009. A requirement for the inclusion of patients with multivessel disease was that nonculprit lesions were treated with the same stent as the culprit lesion. These were strictly consecutive series and each center contributed similar series of both stents. The potential bias by hospital was reduced because an equivalent number of patients was provided by each center for each stent group (around 30 patients per stent group/center).
The only clinical or angiographic exclusion criterion was the presence of cardiogenic shock at the time of the procedure.
Given the retrospective nature of the registry, the procedures were performed according to the local and operator standards and preferences at the time of the intervention. All clinical, angiographic, procedural, and follow-up data were collected in a database specifically designed for the study. One investigator at each center was trained to enter information in the database and perform the follow-up. The follow-up involved reviewing all medical records available in departments, hospitals, and health services; telephone contact with the patient was also requested.
The database was analyzed at the coordination center, Hospital Universitario Marques de Valdecilla, Santander, Spain, by 2 investigators blinded to study group allocation. Events were adjudicated according to the definitions provided in this manuscript. Even though these definitions were accepted and applied by all investigators, the final adjudication of events was conducted by 2 blinded, independent investigators at the ESTROFA study coordination center. These investigators reviewed each event adjudication and the clinical data provided for patients with adjudicated and nonadjudicated events. Additional information was requested and discussed with the corresponding main investigators when any doubts existed and event readjudication was done in order to guarantee a homogeneous adjudication process.
Study Endpoints and DefinitionsThe primary study endpoint was the composite of death, myocardial infarction (MI), and target lesion revascularization (TLR) at 2 years. Secondary endpoints were death, combined endpoint of death and infarction, and incidence of definite or probable thrombosis.
The following major adverse cardiac events were defined: mortality as all-cause death; cardiac death as mortality due to cardiac etiologies such as infarction, heart failure, or stent thrombosis and including any sudden death by undefined cause; and MI as meeting detailed criteria. The MI criteria were the following: a) detection of rise and fall of cardiac biomarkers (preferably troponin) with at least 1 value above the 99th percentile of the upper reference limit, together with evidence of myocardial ischemia with at least 1 of these symptoms: chest pain, electrocardiogram changes (new ST-T changes or new left bundle branch block), or development of pathological Q waves, new regional wall motion, or perfusion abnormalities; b) sudden death involving cardiac arrest, often with previous symptoms suggestive of ischemia, and accompanied by presumably new ST-segment elevation or new left bundle branch block and/or evidence of fresh thrombus in angiography and/or at autopsy (if death occurred before blood samples could be obtained or before cardiac markers appeared in blood), and c) pathological findings of an acute MI.
The TLR was considered as percutaneous revascularization of a stent restenosis lesion including adjacent segments proximal or distal (5mm) to the stent. Target lesion revascularization was also considered as any revascularization surgery for in-stent restenosis. Definite or probable stent thrombosis was determined according to the Academic Research Consortium definitions. Definite stent thrombosis was confirmed by angiography or by pathologic confirmation of acute thrombosis in patients with acute coronary syndromes. Probable stent thrombosis was defined as any unexplained death within 30 days or as target vessel infarction without angiographic confirmation of thrombosis or other identified culprit lesion.
Statistical AnalysisThe estimate of required sample size was based on the results of previous trials with PES in STEMI and multiple non-STEMI trials comparing PES and EES.8,12–15 The observed incidence of the primary outcome for PES at 2 years was 16% to 17% and the expected incidence for EES was 9% to 10%. The registry was originally designed to enroll 800 patients, to ensure a power of 80% to detect this reduction in 2-year incidence of the primary outcome. After the inclusion of > 1000 patients, significant differences were observed between groups, especially in the use of thrombectomy. We performed a propensity-score matching to adjust for differences in clinical, angiographic, and procedural characteristics. The matching procedure was done separately for the cohorts with and without thrombectomy. The ‘psmatching’ custom dialogue was used in conjunction with SPSS version 19. The ‘psmatching’ program performs all analyses in R though the SPSS R-Plugin (version 2.10.1).
This procedure involved 3 stages: a) the propensity scores were estimated using logistic regression, in which the DES type or thrombectomy were used for each matching procedure as the outcome variable and all the covariates as predictors; b) patients were matched using simple 1.1 nearest neighbors matching, imposing a caliper of 0.2 of the standard deviation of the logit of the propensity score to exclude bad matches, and c) a series of model adequacy checks were performed to determine whether balance on the covariates was achieved through the matching procedure.
Continuous variables are presented as mean (standard deviation). Categorical variables are expressed as percentages. Continuous variables were compared with the Student t test if they followed a normal distribution or with Wilcoxon test when not normally distributed (distribution type was assessed with the Kolmogorov-Smirnov test). Categorical variables were compared with the chi-square test or the Fischer exact test as required. Kaplan-Meier curves of event-free survival were obtained for each prespecified group or subgroup and were compared using the log-rank test. We used Cox proportional-hazards regression to determine hazard ratios for selected outcomes and to conduct a subgroup analysis of the primary outcome, using a test of study-group assignment according to subgroup interaction. All variables showing an association with the incidence of major adverse cardiac events in the univariate analysis (P < .1) were included in the model. The hospital and time period for recruitment were also included. A P value of .05 was considered statistically significant. All statistical analyses were performed using SPSS version 19 for Windows.
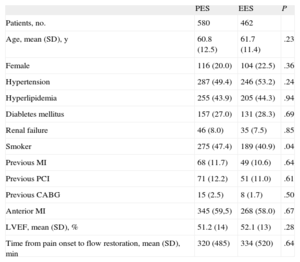
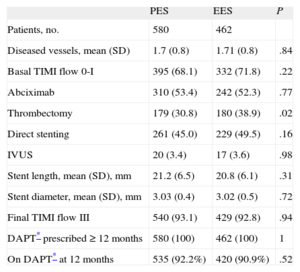
RESULTSA total of 1042 patients, 580 treated with PES and 462 treated with EES, were included in the registry. The clinical and procedural characteristics of both groups are shown in Tables 1 and 2. The groups presented a significant difference in smoking habit and more significantly in the use of thrombus aspiration. Thus, we conducted a propensity-score matching, performed separately in the cohort of 683 patients without thrombectomy and the cohort of 359 patients with thrombectomy. The matching process provided 700 patients, 350 treated with EES and 350 with PES. In each group, 132 (37.7%) patients had thrombectomy. Clinical characteristics are shown in Table 3. Angiographic and procedural characteristics are described in Table 4. No significant differences were observed; both groups were well-balanced for all variables. The follow-up was completed in 694 (99.1%) patients, with 3 patients lost in each group.
Clinical Characteristics
| PES | EES | P | |
| Patients, no. | 580 | 462 | |
| Age, mean (SD), y | 60.8 (12.5) | 61.7 (11.4) | .23 |
| Female | 116 (20.0) | 104 (22.5) | .36 |
| Hypertension | 287 (49.4) | 246 (53.2) | .24 |
| Hyperlipidemia | 255 (43.9) | 205 (44.3) | .94 |
| Diabletes mellitus | 157 (27.0) | 131 (28.3) | .69 |
| Renal failure | 46 (8.0) | 35 (7.5) | .85 |
| Smoker | 275 (47.4) | 189 (40.9) | .04 |
| Previous MI | 68 (11.7) | 49 (10.6) | .64 |
| Previous PCI | 71 (12.2) | 51 (11.0) | .61 |
| Previous CABG | 15 (2.5) | 8 (1.7) | .50 |
| Anterior MI | 345 (59,5) | 268 (58.0) | .67 |
| LVEF, mean (SD), % | 51.2 (14) | 52.1 (13) | .28 |
| Time from pain onset to flow restoration, mean (SD), min | 320 (485) | 334 (520) | .64 |
CABG, coronary artery by-pass graft; EES, everolimus-eluting stent; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PES, paclitaxel-eluting stent; PCI, percutaneous coronary intervention; SD, standard deviation.
Unless otherwise indicated, data are expressed as No. (%) or mean (standard deviation).
Angiographic and Procedural Characteristics
| PES | EES | P | |
| Patients, no. | 580 | 462 | |
| Diseased vessels, mean (SD) | 1.7 (0.8) | 1.71 (0.8) | .84 |
| Basal TIMI flow 0-I | 395 (68.1) | 332 (71.8) | .22 |
| Abciximab | 310 (53.4) | 242 (52.3) | .77 |
| Thrombectomy | 179 (30.8) | 180 (38.9) | .02 |
| Direct stenting | 261 (45.0) | 229 (49.5) | .16 |
| IVUS | 20 (3.4) | 17 (3.6) | .98 |
| Stent length, mean (SD), mm | 21.2 (6.5) | 20.8 (6.1) | .31 |
| Stent diameter, mean (SD), mm | 3.03 (0.4) | 3.02 (0.5) | .72 |
| Final TIMI flow III | 540 (93.1) | 429 (92.8) | .94 |
| DAPT* prescribed ≥ 12 months | 580 (100) | 462 (100) | 1 |
| On DAPT* at 12 months | 535 (92.2%) | 420 (90.9%) | .52 |
DAPT, dual antiplatelet therapy; EES, everolimus-eluting stent; IVUS, intravascular ultrasound; PES, paclitaxel-eluting stent; SD, standard deviation; TIMI, Thrombolysis In Myocardial Infarction.
Unless otherwise indicated, data are expressed as No. (%) or mean (standard deviation).
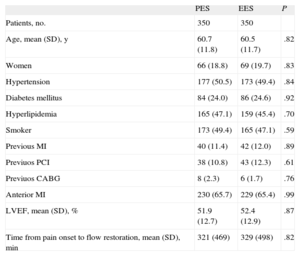
Clinical Characteristics in Propensity Score Matched Groups
| PES | EES | P | |
| Patients, no. | 350 | 350 | |
| Age, mean (SD), y | 60.7 (11.8) | 60.5 (11.7) | .82 |
| Women | 66 (18.8) | 69 (19.7) | .83 |
| Hypertension | 177 (50.5) | 173 (49.4) | .84 |
| Diabetes mellitus | 84 (24.0) | 86 (24.6) | .92 |
| Hyperlipidemia | 165 (47.1) | 159 (45.4) | .70 |
| Smoker | 173 (49.4) | 165 (47.1) | .59 |
| Previous MI | 40 (11.4) | 42 (12.0) | .89 |
| Previuos PCI | 38 (10.8) | 43 (12.3) | .61 |
| Previuos CABG | 8 (2.3) | 6 (1.7) | .76 |
| Anterior MI | 230 (65.7) | 229 (65.4) | .99 |
| LVEF, mean (SD), % | 51.9 (12.7) | 52.4 (12.9) | .87 |
| Time from pain onset to flow restoration, mean (SD), min | 321 (469) | 329 (498) | .82 |
CABG, coronary artery by-pass graft; EES, everolimus-eluting stent; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PES, paclitaxel-eluting stent; PCI, percutaneous coronary intervention.
Unless otherwise indicated, data are expressed as No. (%) or mean (standard deviation).
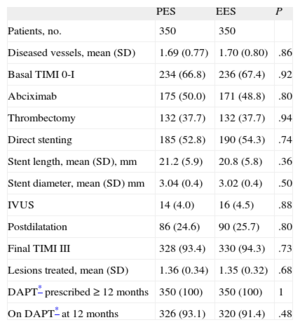
Angiographic and Procedural Characteristics in Propensity Score Matched Groups
| PES | EES | P | |
| Patients, no. | 350 | 350 | |
| Diseased vessels, mean (SD) | 1.69 (0.77) | 1.70 (0.80) | .86 |
| Basal TIMI 0-I | 234 (66.8) | 236 (67.4) | .92 |
| Abciximab | 175 (50.0) | 171 (48.8) | .80 |
| Thrombectomy | 132 (37.7) | 132 (37.7) | .94 |
| Direct stenting | 185 (52.8) | 190 (54.3) | .74 |
| Stent length, mean (SD), mm | 21.2 (5.9) | 20.8 (5.8) | .36 |
| Stent diameter, mean (SD) mm | 3.04 (0.4) | 3.02 (0.4) | .50 |
| IVUS | 14 (4.0) | 16 (4.5) | .88 |
| Postdilatation | 86 (24.6) | 90 (25.7) | .80 |
| Final TIMI III | 328 (93.4) | 330 (94.3) | .73 |
| Lesions treated, mean (SD) | 1.36 (0.34) | 1.35 (0.32) | .68 |
| DAPT* prescribed ≥ 12 months | 350 (100) | 350 (100) | 1 |
| On DAPT* at 12 months | 326 (93.1) | 320 (91.4) | .48 |
DAPT, dual antiplatelet therapy; EES, everolimus-eluting stent; IVUS, intravascular ultrasound; PES, paclitaxel-eluting stent; TIMI, Thrombolysis In Myocardial Infarction.
Unless otherwise indicated, data are expressed as No. (%) or mean (standard deviation).
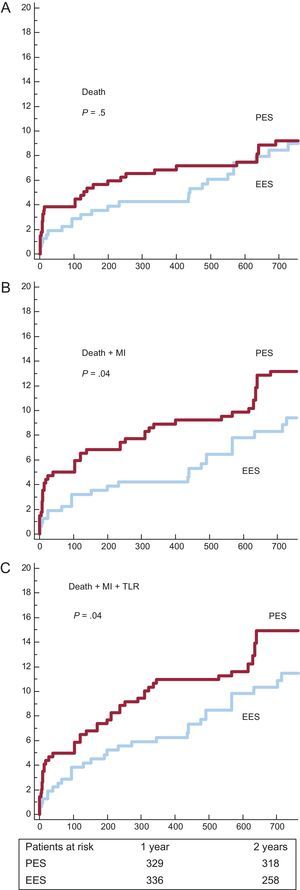
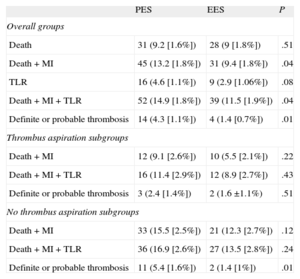
Survival curves and cumulative incidences of major adverse events are shown in Figures 1 and 2 and in Table 5, respectively. The incidence of death was quite comparable, but the incidences of the combined end-points (death-MI and death-MI-TLR) were significantly reduced in the EES group. The risk of definite or probable stent thrombosis was also lower in the EES group.
Survival curves for major acute cardiovascular events. A: incidence of death. B: incidence of death and myocardial infarction. C: incidence of death, myocardial infarction and target lesion revascularization. EES, everolimus-eluting stents; MI, myocardial infarction; PES, paclitaxel-eluting stents; TLR, target lesion revascularization.
Cumulative Incidence of Major Acute Cardiovascular Events at 2 Years
| PES | EES | P | |
| Overall groups | |||
| Death | 31 (9.2 [1.6%]) | 28 (9 [1.8%]) | .51 |
| Death + MI | 45 (13.2 [1.8%]) | 31 (9.4 [1.8%]) | .04 |
| TLR | 16 (4.6 [1.1%]) | 9 (2.9 [1.06%]) | .08 |
| Death + MI + TLR | 52 (14.9 [1.8%]) | 39 (11.5 [1.9%]) | .04 |
| Definite or probable thrombosis | 14 (4.3 [1.1%]) | 4 (1.4 [0.7%]) | .01 |
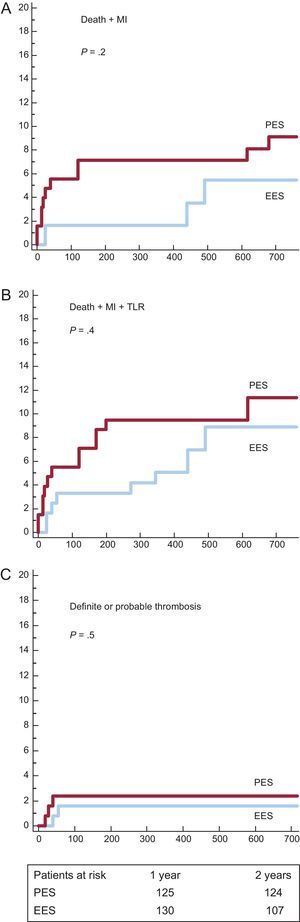
| Thrombus aspiration subgroups | |||
| Death + MI | 12 (9.1 [2.6%]) | 10 (5.5 [2.1%]) | .22 |
| Death + MI + TLR | 16 (11.4 [2.9%]) | 12 (8.9 [2.7%]) | .43 |
| Definite or probable thrombosis | 3 (2.4 [1.4%]) | 2 (1.6 ±1.1%) | .51 |
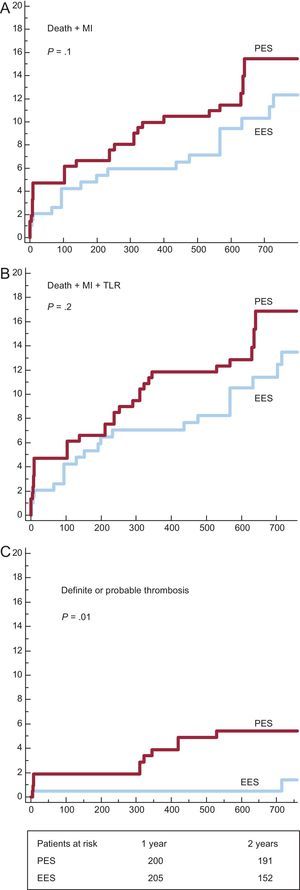
| No thrombus aspiration subgroups | |||
| Death + MI | 33 (15.5 [2.5%]) | 21 (12.3 [2.7%]) | .12 |
| Death + MI + TLR | 36 (16.9 [2.6%]) | 27 (13.5 [2.8%]) | .24 |
| Definite or probable thrombosis | 11 (5.4 [1.6%]) | 2 (1.4 [1%]) | .01 |
EES, everolimus-eluting stent; MI, myocardial infarction; PES, paclitaxel-eluting stent; TLR, target lesion revascularization.
Cumulative incidence is expressed as No. (mean [standard deviation]).
The independent predictors for the combined end-points, death-MI and death-MI-TLR, are listed in Table 6. PES was an independent predictor for both. The use of thrombus aspiration was an independent predictor of death-MI.
Independent Predictors for Major Acute Cardiovascular Events
| HR (95%CI) | P | |
| Death and infarction | ||
| Age | 1.03 (1.01–1.05) | .0087 |
| Diabetes mellitus | 6.34 (3.60–11.16) | .0001 |
| Previous PCI | 2.18 (1.17–4.06) | .0137 |
| LVEF | 0.96 (0.95–0.98) | .0004 |
| Thrombus aspiration | 0.29 (0.14–0.62) | .0015 |
| PES | 3.62 (1.72–7.64) | .0007 |
| Death, infarction and TLR | ||
| Age | 1.04 (1.02–1.07) | .0001 |
| Diabetes mellitus | 5.88 (3.47–9.95) | .0001 |
| Previous PCI | 2.21 (1.27–3.87) | .0052 |
| Smoker | 1.53 (1.08–2.18) | .0160 |
| LVEF | 0.97 (0.95–0.99) | .0036 |
| PES | 2.44 (1.28–4.65) | .0067 |
95%CI, 95% confidence interval; EES, everolimus-eluting stent; HR, hazard ratio; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PES, paclitaxel-eluting stent; TLR, target lesion revascularization.
We analyzed DES subgroups regarding the use of thrombus aspiration. Survival curves and cumulative incidences of major adverse events are shown in Figures 3 and 4 and in Table 5, respectively. In the subgroup with thrombus aspiration (Figure 3), a nonsignificant trend in favor of EES was observed. In the subgroup without thrombectomy (Figure 4), a nonsignificant trend favoring EES was also found for the combined end-points but a significantly lower incidence of stent thrombosis with EES was observed.
Survival curves for major acute cardiovascular events in subgroups with thrombectomy. A: Incidence of death and myocardial infarction. B: incidence of death, myocardial infarction and target lesion revascularization. C: incidence of definite and probable thrombosis EES, everolimus-eluting stents; MI, myocardial infarction; PES, paclitaxel-eluting stents; TLR, target lesion revascularization.
Survival curves for major actue cardiovascular events in subgroups without thrombectomy. A: incidence of death and myocardial infarction. B: incidence of death, myocardial infarction and target lesion revascularization. C: incidence of definite and probable thrombosis. EES, everolimus-eluting stents; MI, myocardial infarction; PES, paclitaxel-eluting stents; TLR, target lesion revascularization.
A significant interaction was found in the subgroups with and without thombectomy in the comparison between PES and EES for the end-point of stent thrombosis (P = .039).
DISCUSSIONThe study findings are the following: a) in this propensity-score-matched registry, the use of EES compared to PES in the STEMI setting was associated with a lower incidence of major adverse cardiac events at 2 years; b) the incidence of definite or probable thrombosis at 2 years was lower with EES, and c) the lower risk of thrombosis with the EES seems to be greater in the absence of thrombectomy.
Second-generation DES and particularly EES have shown greater efficacy and a better safety profile than PES in both clinical trials and multiple registries.12–15 Therefore, it is important to assess the outcomes of these new stents in the STEMI setting. However, very few published studies have done so. In fact, the last European guidelines for STEMI management recommend DES over bare-metal stents in this clinical setting,25 but no distinction is made between first- and second-generation DES.26
The EXAMINATION16 trial compared EES to bare-metal stents without finding any differences in the primary endpoint at 1 year, but found a significantly lower incidence of thrombosis in the EES group. Another recently published trial compared everolimus-eluting and sirolimus-eluting stents in 625 patients with STEMI, showing noninferiority for EES with suggestive superiority.17 A subanalysis of the single-center COMPARE27 study analyzed the differences between PES and EES at 2 years in 452 patients with STEMI. This study found a lower incidence of the primary endpoint, death, infarction, and TLR with EES. This difference might be mostly explained by the differences in TLR. Thrombosis was significantly lower with EES (1% vs 3%). In a meta-analysis of 22 trials comparing different DES or DES with bare-metal stents in STEMI, the most favorable profile corresponded to the EES.28 Our multicenter all-comers registry with series of matched patients found some results in line with this latter study. The better safety profile found for EES agrees with that observed in multiple trials and registries conducted in patients outside the context of STEMI.12–15 As already shown in the previously mentioned EXAMINATION trial,16 the EES safety profile might be even superior to that of bare-metal stents.
This better performance of EES could be explained by a more complete and homogeneous endothelization provided by these stents, which could also be related to the following factors: a) smaller strut thickness; b) the fluorinated polymer performance, very biocompatible with lower thrombogenicity, lower inflammation, and a more attenuated platelet activation, and c) the different cellular and molecular actions of the macrocyclic lactone group (“-limus drugs”) and paclitaxel, as well as the different-release kinetics might play a role.29,30 In a study with 35 patients with STEMI randomized to EES and sirolimus-eluting stent, the assessment with optical coherence tomography at 7 months showed better arterial healing response with EES.31
Regarding the very low figures for TLR, it is clinically less likely to need a new revascularization of a vessel that supplies an infarcted myocardium. In fact, the incidences of TLR are generally lower in the STEMI setting than in other settings. Thus, differences in TLR between DES and bare-metal stents are smaller after infarction procedures and large patient samples should be required to find a significant difference. For example, PES were not superior to bare-metal stents in TLR in the PASSION trial7 with 619 patients but they were superior in the HORIZONS trial8 with 3006 patients. However, in the EXAMINATION trial,16 EES did not achieve a significantly lower incidence of TVR at 1 year (7% with bare-metal stents and 3.9% with EES), but this could be explained by the good performance of the bare-metal stents used (thin cobalt-chromium stents).
The incidence of thrombosis and TLR with PES in our study was comparable to those observed in the HORIZONS trial8 or in the STEMI subgroup of COMPARE.27 Moreover, the incidence of thrombosis and TLR with EES in our study was in the same range observed with these stents in the EXAMINATION trial16 or in the substudy of the COMPARE trial.27
Special consideration should be given to the use of thrombectomy, which has not been specifically analyzed in the abovementioned studies. It is of note that, although patients treated with EES tended to achieve better outcome regardless to the use of thrombectomy, the lower risk of thrombosis with EES was more evident in cases without thrombectomy.
The risk of thrombosis with first-generation DES in STEMI is related to the thrombus burden.24 The findings in our study suggest that in the absence of thrombus aspiration a potential larger thrombus burden underlying the stent increased the risk of thrombosis with PES but not with EES, maybe due to the mentioned protective factors linked to the design of the latter stent.
Several clinical studies and meta-analyses have suggested a benefit from thrombus aspiration.18–21 However, 2 recently published studies found no benefit associated with thrombus aspiration, either in terms of infarct size reduction or in survival advantage at 30 days.22,23
In our study, the selective use of manual-aspiration thrombectomy was an independent predictor of death and infarction. The benefit of thrombectomy could be explained by its selective application and could be related to a lower residual thrombus burden in the vessel and beneath the stent, along with a more appropriate microvascular reperfusion. Thrombus removal from the lesion prior to stent implantation may decrease the chance for late incomplete apposition and may increase the pace of neo-intimal coverage. This could be a mechanistic explanation for the interaction between thrombus aspiration and the DES model, since PES has shown to be more prone to present delayed coverage and late malapposition and these are well known risk factors for late stent thrombosis.
LimitationsThis is a retrospective comparative registry with different treatment periods and baseline differences between groups. This entails several limitations, mainly the burden of potential confounding factors, which are not always sorted out even after the adjustment with matched analysis such as propensity score. The gap between time periods for recruitment of both stent groups implies a limitation because some differences in medical management could have influenced the outcomes. The consecutiveness of the inclusion was not externally verified and this was left to the investigator's commitment.
Given the retrospective nature of the study, the procedures were performed according to the local and operator standards and preferences at the time of the intervention. Nevertheless, these standards were comparable between centers. Having said this, the inclusion in the multivariate analysis of the time period for enrollment and the hospital did not affect the results and these variables were not independent predictors for outcomes.
The study was not sufficiently powered for the endpoints. Therefore, these results should be considered as hypothesis generators and larger randomized trials with EES or with thrombectomy will be needed in the STEMI setting.
CONCLUSIONSThe results of this retrospective multicenter registry suggest that the use of EES in STEMI could be associated with better clinical outcomes compared to the use of PES. The reduction of thrombosis risk with EES could be more relevant in the absence of thrombectomy.
CONFLICTS OF INTERESTJose M. de la Torre Hernández has received investigation grant and he is consultant from Boston Scientific and Abbott Vascular.