Cardiovascular magnetic resonance (CMR) imaging has been increasingly used for testing of translational and clinical trial surrogate endpoints in cardioprotective therapies. While the JACC Scientific Expert Panel provides imaging technique recommendations and standardization1, postprocessing and analysis methods vary institutionally. Moreover, most previous CMR postprocessing comparison and software testing data stem from human hearts. Pig hearts largely resemble their human counterparts. However, pigs have cone-shaped chests and higher resting heart rates than humans. Medis Suite (QMass MR v.3.2.60.4, The Netherlands) and CVI42 (v.5.11, Circle Cardiovascular Imaging, Canada) are among the most widely used scanner-independent CMR postprocessing software programs. However, their interchangeability to assess anatomical and functional parameters in preclinical models has not been tested. We aimed to compare Medis Suite and CVI42 readouts in a pig model of experimentally induced closed-chest acute myocardial infarction (MI). All procedures were authorized by the Animal Experimental Committee (#5601) of the local government.2 We assessed anatomical and functional parameters in randomly selected 28 LandracexLarge white female pig datasets, which included baseline (before MI), early- (3 days post-MI), and late- (42 days post-MI) remodeling phase scans.2 In addition, 25 of 28 scans included a dobutamine stress study (5-10-20-30μg/kg/min of i.v. dobutamine at 3-minute intervals to elevate heart rate by 30-50%) using the volumetric module. To exclude interobserver- and experience-related variabilities, all images were blindly assessed by a Level 3 accredited operator. Due to animals’ cardiac orientation, the quality of semi- and fully-automated ventricular contour segmentation was suboptimal in both products; thus, manual contouring was chosen.
The following were recorded: left ventricular (LV) end-diastolic volume, LV end-systolic volume, LV stroke volume, LV ejection fraction (LVEF), LV mass, right ventricular (RV) end-diastolic volume, RV end-systolic volume, RV stroke volume, and RV ejection fraction. Edema, microvascular obstruction (MVO), and necrosis mass were assessed on T2 short-tau inversion recovery and T1 inversion recovery sequences at early (1minute) and late (10minutes) gadolinium phases, respectively. On Medis Suite, we used visual assessment-defined manual planimetry on the volumetry module to draw the region(s) of interest (the late gadolinium enhancement [LGE] volume was multiplied by the myocardial density of 1.055g/mL), and the full-width half-max (FWHM) technique, using the tissue characterization module with semiautomatic pixel value segmentation. Of note, MVO measurement on Medis Suite FWHM is planimetry-based, as the region of interest is user-defined without semiautomatic segmentation. On CVI,42 as planimetry was unavailable for tissue characterization, we used FWHM. The day 42 LGE data were correlated with infarct size assessed by triphenyl tetrazolium chloride (TTC) staining.2
To detect low and strong correlation variables, accepting an alpha risk of 0.05 and a beta risk of 0.2 in 2-sided tests, 28 datasets were needed to detect a correlation coefficient of 0.51. A dropout rate of 0% was anticipated. After normal distribution testing (Shapiro-Wilk), data were analyzed for correlation by the Pearson or Spearman tests, when appropriate. The Wilcoxon matched-pairs signed-rank test and the paired t-test were used to compare groups, matched as pair measurements of the same subject. For groups not following a normal distribution, equivalent nonparametric tests were performed.
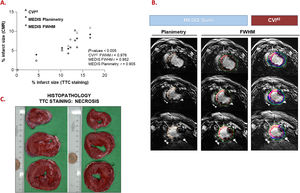
The 28 datasets consisted of 3 baseline, 15 early remodeling, and 10 late remodeling phases; among the latter, 8 had histopathological analysis. Dobutamine stress was available in 25 datasets (89%). The 2 products provided similar data for biventricular volumes and LVEF, with significantly related correlation curves between measurements and Bland-Altman plots, showing only a minor systematic measurement error at rest and stress (P=nonsignificant). Only LV mass showed a mean difference of 10.58g at stress (figure 1). The data were very similar in all structural parameters; as such, using planimetry and FMWH, we detected a high correlation between software in the necrosis, edema, and MVO quantification (P=non-significant), and Bland-Altman plots showed near-zero systematic differences for the 3 tested parameters (figure 1). LGE quantification agreed better on planimetry on Medis Suite and CVI42 compared with FWHM on Medis Suite vs CVI42, and planimetry vs FWHM on Medis Suite alone. FWHM on both showed a better correlation with histopathology (TTC staining) than planimetry. However, CVI42 FWHM performed better than Medis Suite FWHM (figure 2).
Bland-Altman graphs (Medis Suite-CVI42) vs average to analyze systematic differences at rest (A) and stress (B) of the left ventricular mass. C: Bland-Altman graphs to analyze systematic differences in the tissue characterization parameters between different methods. Dotted black lines indicate mean difference (bias; see also value in bold) and dashed grey lines indicate limits of agreement 95%. FWHM, full-width half maximum; LV, left ventricle; MVO, microvascular obstruction.
Correlation between the CMR-derived necrosis percentage (Medis Suite and CVI42) and histopathology (TTC staining) on the 8 datasets (A). Representative same-day CMR (B) and histopathology (C) images from the same animal. CMR, cardiovascular magnetic resonance; FWHM, full-width half maximum.
Our results of volumetry comparison align with previous human data.3 Likewise, the LV mass variability agrees with human studies,4,5 supporting the contouring bias and suggesting that LV mass and its derivates (eg, fibrosis percentage) may be less reliable in tachycardia with hyperdynamic ventricles. While LV mass is rarely calculated under stress, the contouring variability in a hyperdynamic LV may be reduced by using the same software. As tissue characterization techniques have evolved, most CMR infarct validation studies in animal models (mainly dogs) are from the 1980s-1990s.6 Despite different available techniques, we report good reproducibility in all 3 tissue parameters in pigs. LGE correlated best between planimetry on Medis Suite and FWHM on CVI42. However, direct comparison between CMR scar size and TTC staining (both performed on day 42) revealed better FWHM performance in histopathological correlation vs planimetry, particularly on CVI42. Small software-specific differences in semiautomatic segmentation may have contributed to this finding, indicating the need for further histopathology-validated studies for technique standardization.
In conclusion, both software tools can be used interchangeably for biventricular volumes, edema, and MVO. A single product should be considered for LV mass and necrosis follow-up. Because CMR use in experimental disease models has been increasing along with ever-evolving markers and postprocessing techniques, researchers should evaluate their postprocessing methods carefully to deliver reproducible results for a truly reliable bench-to-bedside translation.
FUNDINGThis work was supported by PGC 2018-094025-B-I00 to G. Vilahur; and PID2019-107160RB-I00 to L. Badimon; funded by MCIN/AEI/10.13039/501100011033 and Fondo Europeo de Desarrollo Regional (FEDER) A way of making Europe; Instituto de Salud Carlos III (CIBERCV CB16/11/00411 to L. Badimon); TERCEL RD16/0011/018 to L. Badimon; the Generalitat of Catalunya-Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat (2017SGR1480 to L. Badimon) and 2016PROD00043 (Agencia Gestión Ayudas Universitarias Investigación: AGAUR); and the Fundación Investigación Cardiovascular-Fundación Jesus Serra for their continuous support. This work is part of the Autonomous University of Barcelona requirement for the Doctorate in Medicine (M. Radiké).
AUTHORS’ CONTRIBUTIONSM. Radiké: conception and design, data analysis and interpretation, manuscript drafting. S. Ben-Aicha: data analysis and interpretation; M. Gutiérrez: manuscript drafting and data interpretation. A. Hidalgo: conception and design; final manuscript approval. L. Badimon and G. Vilahur: conception and design; critical revision for important intellectual content; final manuscript approval; both authors are corresponding authors.
CONFLICTS OF INTERESTNone to declare.
We gratefully acknowledge the valuable help and support of M.A. Canovas, P. Catalina, and J. Moreno with animal handling and of A. Nuñez and J. Exposito with CMR acquisition and their proper conduct of all the experimental, molecular, and technical work.