Catecholaminergic polymorphic ventricular tachycardia (VT) is a cardiac conduction disorder whereby changes in intracellular calcium regulation increase susceptibility to ventricular arrhythmias with a consequent risk of sudden death despite a structurally normal heart. Affected individuals usually experience exercise-induced syncope and the arrhythmia is characteristically a bidirectional VT.1
The surface electrocardiogram usually shows no abnormalities and diagnosis is complex and based on 24-hour electrocardiographic monitoring and exercise testing. Epinephrine or isoproterenol tests are also useful. Even so, some cases remain undiagnosed despite a clinical manifestation in the form of serious ventricular fibrillation (VF), initially classed as idiopathic.2,3 Recently, genetic testing has become available. Mutations have been identified in up to 5 genes: the ryanodine receptor (RyR2) gene, which is the most common genetic abnormality, cardiac calsequestrin (CASQ2) gene,1 genes coding tight junction proteins, calmodulin gene, and KCNJ2.
The aim of the present study was to investigate the clinical characteristics and the usefulness of different diagnostic tests in a series of 9 patients with catecholaminergic polymorphic VT.
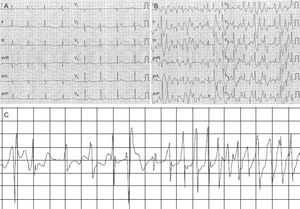
The reason for studying these 9 patients (mean age, 16 [standard deviation, 11.2] years; 55.5% women) was syncope in 7, resuscitation after VF in 1, and pathologic electrocardiogram with multiple ventricular extrasystoles in 1. VF was reported as part of the clinical course in 3 patients (33.3%), all before starting treatment with beta-blockers and after syncope. After therapy was started, no further arrhythmic events were reported except in patient 2, who had an appropriate shock on the only day he did not take beta-blockers (Figure).
Patient 2. A: Baseline electrocardiogram of the patient. B: Electrocardiogram after intravenous infusion of adrenaline. C: Episode of ventricular fibrillation with onset after a series of bidirectional ventricular tachycardias recorded by the implantable cardioverter-defibrillator after temporary interruption (of 1 day) in beta-blocker treatment.
No pathologic findings were reported in the electrocardiogram in 55.5% of the patients (Table). The mean QTc interval was 385 (SD, 26) ms (range, 347-425ms) and the mean U-wave voltage was 0.14 (SD, 0.12) mV.
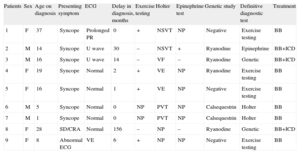
Clinical and Genetic Characteristics of the 9 Patients Included in the Series
| Patients | Sex | Age on diagnosis | Presenting symptom | ECG | Delay in diagnosis, months | Exercise testing | Holter | Epinephrine test | Genetic study | Definitive diagnostic test | Treatment |
| 1 | F | 37 | Syncope | Prolonged PR | 0 | + | NSVT | NP | Negative | Exercise testing | BB |
| 2 | M | 14 | Syncope | U wave | 30 | – | NSVT | + | Ryanodine | Epinephrine | BB+ICD |
| 3 | M | 16 | Syncope | U wave | 14 | – | VF | – | Ryanodine | Genetic | BB+ICD |
| 4 | F | 19 | Syncope | Normal | 2 | + | VE | NP | Ryanodine | Exercise testing | BB |
| 5 | F | 16 | Syncope | Normal | 1 | + | VE | NP | Negative | Exercise testing | BB |
| 6 | M | 5 | Syncope | Normal | 0 | NP | PVT | NP | Calsequestrin | Holter | BB |
| 7 | M | 1 | Syncope | Normal | 0 | NP | PVT | NP | Calsequestrin | Holter | BB |
| 8 | F | 28 | SD/CRA | Normal | 156 | – | NP | – | Ryanodine | Genetic | BB+ICD |
| 9 | F | 8 | Abnormal ECG | VE | 6 | + | NP | NP | Negative | Exercise testing | BB |
BB, beta-blockers; CRA, cardiorespiratory arrest; ECG, electrocardiogram; F, female; ICD, implantable cardioverter device; LV, left ventricular; LVEF, left ventricular ejection fraction; M, male; NSVT, nonsustained ventricular tachycardia; PVT, polymorphic ventricular tachycardia; SD, sudden death; VE, ventricular extrasystoles; VF, ventricular fibrillation.
The complementary test that completed diagnosis was exercise testing in 44.4%, 24-hour Holter monitoring in 22%, epinephrine test in 11.1%, and genetic testing (including RyR2 and CSQ2) in 40%. Although symptoms were triggered by substantial physical or psychological stress in all patients, some patients did not have pathologic values in exercise testing or in the epinephrine test (Table). Interestingly, exercise testing was inconclusive for diagnosis in 3 of 7 patients (42.8%). These patients required the epinephrine or genetic tests (Table). In patients 6 and 7, exercise testing was not performed because bidirectional VT had been detected in the 24-hour Holter monitoring. In all 7 of the exercise tests performed, the patients attained submaximal heart rates for their age. Therefore, the cumulative diagnostic rate for each diagnostic step was 6 of 9 cases for the first step of exercise testing or 24-hour Holter monitoring, 1 of 3 for the second study of the epinephrine test, and 2 of 2 for the genetic test, performed last after negative results in all the previous tests. The overall sensitivity of the genetic test (prevalence of RyR2 and CSQ2 mutations) was 6 of 9 (66.6%), that is, similar to previous studies.
The time to definitive diagnosis after symptom onset is an important parameter. In our series, the mean delay in diagnosis was 23.2 months (median, 2 months; range 0-156 months). During the time when diagnosis was delayed, clinical events occurred in 3 patients. Some of these events such as VF (1 event) or syncope (2 events) were serious.
It is appropriate to note, as proposed by Kraha et al in their studies of idiopathic VF2, that an extensive diagnostic workup was required in our study aimed at detecting subclinical conduction disorders. This diagnostic workup included genetic testing. In this respect, patients 2 and 3 were of particular interest.
Patient 2 attended the clinic as an 11-year-old boy with exercise-induced syncope. The results of all conventional tests were normal, and therefore an implantable Holter device was deployed and polymorphic VT was detected. Years later, on application of a diagnostic protocol for patients with idiopathic VF, which included pharmacologic and genetic tests,2 the epinephrine test3 detected bidirectional VT consistent with polymorphic catecholaminergic VT (Figure).
Patient 3 is a 16-year-old boy who experienced syncope while swimming in a swimming pool. Complementary tests were negative (Table). Within a year, he had an episode of VF. The epinephrine test was negative but the genetic test was positive for the RyR2 gene, with a heterozygous missense mutation K337N/g398923A>C. The same mutation was also detected in his father and sister.
As reflected in our series of patients, catecholaminergic polymorphic VT is a diagnostic challenge, although early detection is necessary due to the high risk of sudden death in untreated patients and the good response to beta-blockers.1 The common denominator in our patients was the triggering of syncopes or ventricular arrhythmias by exercise or psychological stress. This condition should be considered even when conventional tests yield negative results. Performing genetic tests may be very useful in these patients and help establish diagnosis, thus ensuring selection of the appropriate treatment, which, at times, is guided by the actual mutation detected.2,4