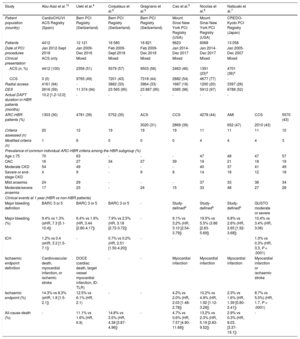
In patients undergoing percutaneous coronary intervention (PCI), those presenting with acute coronary syndrome (ACS) are at higher risk of thrombotic events than those presenting with chronic coronary syndrome (CCS). To mitigate this risk, treatment with more potent and longer duration dual antiplatelet therapy (DAPT) is recommended.1 However, this raises concern about an increased risk of bleeding, particularly in patients already considered to be at high bleeding risk (HBR).
To define HBR in ACS patients undergoing PCI, European practice guidelines recommend the use of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria or a PRECISE-DAPT score of ≥ 25.2 The ARC-HBR arbitrarily defines HBR as a Bleeding Academic Research Consortium (BARC) 3 or 5 bleeding rate of ≥ 4% or an intracranial hemorrhage (ICH) rate of ≥ 1% at 1 year.3 Based on a literature review and expert consensus, 20 risk factors for bleeding were classified into major or minor criteria according to these cutoffs, depending on the associated bleeding risk. A major criterion was defined as any criterion which, in isolation, is associated with the above event rates and a minor criterion was defined as any criterion which, in isolation, is associated with an increased bleeding rate but with a BARC 3 or 5 bleeding rate of <4% at 1 year. Patients who meet ≥ 1 major criterion or ≥ 2 minor criteria are considered to be at HBR. Classification into major or minor criteria was done according to trial and registry data derived from mixed CCS and ACS populations. In addition, previous large-scale studies validating the use of these criteria did so predominantly in mixed CCS and ACS populations from PCI registries using data from the Bern PCI registry in 3 studies, from Mount Sinai New York PCI Registry in 2 studies, and from the CREDO-Kyoto registry in 1 study (table 1).4–9
Studies validating the ARC-HBR criteria in real-world PCI registries
| Study | Abu-Assi et al.10 | Ueki et al.4 | Corpataux et al.6 | Gragnano et al.9 | Cao et al.5 | Nicolas et al.8 | Natsuaki et al.7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Patient population (country) | CardioCHUVI ACS Registry (Spain) | Bern PCI Registry (Switzerland) | Bern PCI Registry (Switzerland) | Bern PCI Registry (Switzerland) | Mount Sinai New York PCI Registry (USA) | Mount Sinai New York PCI Registry (USA) | CREDO-Kyoto PCI Registry (Japan) | ||
| Patients | 4412 | 12 121 | 16 580 | 16 821 | 9623 | 6068 | 13 058 | ||
| Date of PCI procedures | Jan 2012-Sept 2018 | Jan 2009-Dec 2016 | Feb 2009-Sept 2018 | Feb 2009-Dec 2018 | Jan 2014-Dec 2017 | Jan 2014-Dec 2017 | Jan 2005-Dec 2007 | ||
| Clinical presentation | ACS only | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed | ||
| ACS (n, %) | 4412 (100) | 2356 (51) | 9379 (57) | 9503 (56) | 2463 (46) | 1391 (23)a | 4701 (36)a | ||
| CCS | 0 (0) | 9765 (49) | 7201 (43) | 7318 (44) | 2882 (54) | 4677 (77) | - | ||
| Radial access | 4161 (94) | - | 3882 (33) | 3964 (33) | 1687 (18) | 1200 (20) | 3397 (26) | ||
| DES | 2616 (59) | 11 374 (94) | 23 565 (95) | 23 887 (95) | 9385 (98) | 5912 (97) | 6788 (52) | ||
| Actual DAPT duration in HBR patients (months) | 10.2 [1.2-12.0] | - | - | - | - | - | - | ||
| ARC-HBR patients (%) | 1303 (30) | 4781 (39) | 5752 (35) | ACS | CCS | 4278 (44) | AMI | CCS | 5570 (43) |
| 3020 (31) | 2869 (39) | 652 (47) | 2010 (43) | ||||||
| Criteria assessed (n) | 20 | 12 | 19 | 19 | 19 | 11 | 11 | 11 | 10 |
| Modified criteria (n) | 1 | 6 | 0 | 0 | 0 | 4 | 4 | 4 | 3 |
| Prevalence of common individual ARC-HBR criteria among the HBR subgroup (%) | |||||||||
| Age ≥ 75 | 70 | 63 | - | - | - | 47 | 48 | 47 | 57 |
| OAC | 16 | 27 | 34 | 27 | 39 | 19 | 21 | 18 | 19 |
| Moderate CKD | 54 | 49 | - | - | - | 40 | 37 | 41 | 49 |
| Severe or end-stage CKD | 4 | 9 | - | 9 | 8 | 14 | 18 | 12 | 18 |
| Mild anaemia | 24 | 29 | - | - | - | 37 | 33 | 38 | 34 |
| Moderate/severe anaemia | 17 | 23 | - | 24 | 15 | 33 | 48 | 27 | 28 |
| Clinical events at 1 year (HBR vs non-HBR patients) | |||||||||
| Major bleeding definition | BARC 3 or 5 | BARC 3 or 5 | BARC 3 or 5 | - | Study-definedb | Study-definedb | Study-definedb | GUSTO moderate or severe | |
| Major bleeding (%) | 9.4% vs 1.3% (sHR, 7.3 [5.1-10.4]) | 6.4% vs 1.9% (HR, 3.44 [2.80-4.17]) | 7.9% vs 2.5% (HR, 3.18 [2.72-3.72]) | - | 9.1% vs 3.2% (HR, 3.10 [2.54-3.79]) | 19.5% vs 5.5% (3.86 [2.63-5.69]) | 6.8% vs 2.6% (HR, 2.65 [1.92-3.68]) | 10.4% vs 3.4% (HR, 3.06) | |
| ICH | 1.2% vs 0.4 (sHR, 3.3 [1.5-7.1]) | - | 0.7% vs 0.2% (HR, 2.51 [1.50-4.20]) | - | - | - | - | 1.0% vs 0.3% (HR, 3.3, P < .0001) | |
| Ischaemic endpoint definition | Cardiovascular death, myocardial infarction, or ischemic stroke | DOCE (cardiac death, target vessel myocardial infarction, ID-TLR) | - | - | Myocardial infarction | Myocardial infarction | Myocardial infarction | Myocardial infarction or ischaemic stroke | |
| Ischaemic endpoint (%) | 14.3% vs 8.3% (sHR, 1.8 [1.5-2.1]) | 12.5% vs 6.1% (HR, 2.1) | - | - | 4.2% vs 2.0% (HR, 2.03 [1.48-2.78]) | 10.2% vs 4.9% (HR, 1.92 [1.12-3.28]) | 2.3% vs 1.6% (HR, 1.39 [0.80-2.41]) | 8.7% vs 5.0%) (HR, 1.7, P < .0001) | |
| All-cause death (%) | - | 11.1% vs 1.6% (HR, 6.9) | 14.8% vs 3.5% (HR, 4.38 [3.87-4.96]) | - | 4.7% vs 0.6% (HR, 7.57 [4.90-11.68]) | 13.2% vs 2.3% (HR, 5.19 [2.83-9.52]) | 2.9% vs 0.3% (HR, 8.03. [3.37-19.1]) | - | |
ACS, acute coronary syndrome; AMI, acute myocardial infarction; ARC-HBR, Academic Research Consortium for High Bleeding Risk; BARC, Bleeding Academic Research Consortium; CCS, chronic coronary syndrome; CKD, chronic kidney disease; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; HBR, high bleeding risk; HR, hazard ratio; ICH, intracranial hemorrhage; ID-TLR, ischemia-driven target lesion revascularisation; OAC, oral anticoagulation therapy; OR, odds ratio; PCI, percutaneous coronary intervention; sHR, subhazard ratio.
In-hospital and post-discharge bleeding up to 1 year after PCI. In-hospital bleeding was defined according to the National Cardiovascular Data Registry CathPCI Registry definition as a hemoglobin drop of ≥ 3 g/dl, a hemoglobin drop requiring blood transfusion, or bleed requiring intervention at the bleeding site. Bleeding after discharge was defined as any bleed requiring hospitalization or blood transfusion.
With this in mind, in a recent article published in Revista Española de Cardiología, Abu-Assi et al.10 investigate the validity of the ARC-HBR criteria in an ACS population who underwent PCI followed by DAPT (n=4412) and compared the ARC-HBR and PRECISE-DAPT definitions of HBR with respect to their ability to predict bleeding events, using retrospective data from the CardioCHUVI (Cardiología del Complejo Hospitalario Universitario de Vigo) ACS registry. The investigators found that ARC-defined HBR patients compared with non-HBR patients had significantly higher rates of major bleeding at 1 year and that the presence of increasing numbers of criteria in ARC-HBR patients was associated with a corresponding increase in bleeding rates. These findings corroborate the findings of previous validation studies in mixed CCS and ACS populations.4,5,7 In addition, rates of BARC 3 or 5 bleeding and ICH at 1 year met the cutoffs proposed by the ARC-HBR. In addition, bleeding rates associated with all but 4 of the 20 criteria satisfied the predefined ARC-HBR cutoff (a BARC 3 or 5 bleeding rate of ≥ 4% at 1 year for an isolated major criterion or an elevated bleeding rate of <4% for an isolated minor criterion). Of the 4 that did not, their prevalence was very low (ranging from 0.2% to 1.8% of the ARC-HBR population), thus precluding their comprehensive assessment. Finally, compared with the PRECISE-DAPT score, classification according to the ARC-HBR definition resulted in a markedly lower prevalence of HBR patients (30% vs 40%) and more accurate prediction of BARC 3 or 5 bleeding at 1 year, leading the authors to conclude that the ARC system is a more accurate classifier of HBR than the PRECISE-DAPT score.
The authors should be commended for reporting what is, to our knowledge, the first large-scale study validating use of the ARC-HBR criteria in a purely ACS population that includes the full spectrum of ACS presentations. Important strengths of this analysis include the use of BARC 3 or 5 bleeding as the definition of major bleeding, as per the ARC-HBR definition; investigation of ICH rates in addition to major bleeding, and retrospective adjudication, rather than omission or modification, of criteria that were not readily available in the database used. All 20 criteria were assessed, with modification of only 1 (chronic nonsteroidal anti-inflammatory drugs or steroid use). In addition, the high rate of radial access (> 90% of procedures compared with only one third of procedures in the Bern PCI registry and one fifth of procedures in the Mount Sinai registry) increases external validity for contemporary practice. On the other hand, the generalisability of results is somewhat limited by the single-center nature of the study and the fact that in the HBR group, the implanted stent was a drug-eluting stent (DES) in only 48% of patients. Even among non-HBR patients, only 64% were treated with DES. This practice is at odds with current European clinical practice guidelines, which, since 2018, recommend DES as the stent of choice in all patients undergoing PCI.1 While this may be partly explained by the fact that the PCI procedures were performed between January 2012 and September 2018, validation studies from the Bern and Mount Sinai PCI registries reported DES use in ≥ 95% of patients using data of a similar age, which would suggest that this practice largely reflects institution-specific protocols at that time.
It is notable that the prevalence of ARC-defined HBR patients in this population was markedly lower (30%) than that in previous studies of mixed CCS and ACS populations (35%-39% in the Bern PCI registry, 44% in the Mount Sinai registry, and 43% in the CREDO-Kyoto registry).4–7 There are 2 possible explanations for this. First, there may be a lower prevalence of ARC-HBR patients among those presenting with ACS compared with CCS. Second, use of modified ARC-HBR criteria in some previous studies may have overestimated the prevalence of HBR patients. The former explanation is supported by findings from the Bern PCI registry, in which the reported prevalence of ARC-HBR patients was 31% in ACS patients compared with 39% in CCS patients, mainly because ACS patients were younger, with a markedly lower rate of oral anticoagulation use.9 The converse was shown, however, in a study conducted at Mount Sinai, with a reported prevalence of 47% vs 43% in acute myocardial infarction and CCS patients, respectively.8 The exclusion of patients with unstable angina and the modification of some ARC-HBR criteria in the Mount Sinai study might help to explain this reverse trend. The latter also likely explains the higher prevalence of HBR patients overall. Only 11 of the 20 criteria, 4 of which were modified, were adjudicated in the Mount Sinai study compared with 19 unmodified criteria in both the study by Abu-Assi et al. and the Bern study. Substituting the proposed criteria with less specific, modified criteria (eg, “history of any gastrointestinal bleeding” instead of “spontaneous nonintracranial bleeding requiring hospitalization or transfusion 6 to 12 months prior to PCI”) inevitably results in misclassification of a higher proportion of patients as HBR.
Consistent with other validation studies, the most prevalent ARC-HBR criteria in the study by Abu-Assi et al. were age, chronic kidney disease, anemia, and oral anticoagulation, although there was a higher prevalence of elderly patients and a lower prevalence of anemia in the study by Abu-Assi et al.10 It is notable that oral anticoagulation, in isolation, was associated with a BARC 3 or 5 bleeding rate of 5.4% at 1 year compared with only 2.5% in the Bern PCI registry.4 Oral anticoagulation was combined with DAPT (triple therapy) for a median duration of 1.9. months in the study by Abu-Assi et al.,10 whereas no information on DAPT duration or adherence was provided in the latter registry, making comparison difficult. It is also noteworthy that age ≥ 75 years, in isolation, was associated with a BARC 3 or 5 bleeding rate of only 0.7% in the study by Abu-Assi et al.10 While the expected bleeding rate with a minor criterion is <4%, this rate seems lower than expected, particularly in the context of a BARC 3 or 5 bleeding rate of 1.3% in other non-HBR patients (with 0 or 1 minor criterion). The reason for this is not clear.
While higher absolute rates of bleeding are to be expected in ACS compared with CCS patients because of more aggressive antithrombotic treatment,8 it is noteworthy that the relative increase in bleeding rates between HBR and non-HBR patients was more pronounced in the study by Abu-Assi et al.10 compared with studies in mixed populations. There was a 7-fold increase in BARC 3 or 5 bleeding compared with a roughly 3-fold increase in major bleeding in previous studies, irrespective of the bleeding definition used.11 A study from Mount Sinai had consistent findings, although the difference was not as marked: the hazard ratio for bleeding events in HBR vs non-HBR patients was 3.86 in ACS patients compared with 2.65 in CCS patients.8 In contrast, the almost doubling of ischemic events in HBR vs non-HBR patients in the study by Abu-Assi et al. is in line with previous studies in mixed ACS and CCS populations,11 although studies have used different definitions for ischemic outcomes.
Taking all validation studies into consideration, it seems that, compared with CCS patients, a lower proportion of ACS patients may be at HBR, but when these patients do bleed, they do so at a disproportionately higher rate compared with non-HBR patients than is seen in CCS. Whether this is partly caused by some intrinsic systemic inflammatory process at the time of the acute event, or purely by extrinsic factors, including differences in procedural and antithrombotic therapies, is poorly understood. For example, in the Bern PCI registry, patients who presented with ACS compared with CCS had higher rates of femoral access, invasive hemodynamic support, and staged PCI, as well as more potent periprocedural and maintenance antiplatelet therapies, for a longer duration.9 In addition, for reasons that are not clear, in the study by Abu-Assi et al.,10 the rate of femoral access was significantly higher in HBR than in non-HBR patients, a finding that was also observed in the Bern, Mount Sinai and CREDO-Kyoto registries.6–8
One thing is certain: these extrinsic–or iatrogenic–factors are potentially modifiable. Bleeding avoidance strategies, such as default use of radial access, dose-adjustment of intraprocedural anticoagulation, and liberal use of proton pump inhibitors should be given particular attention in ACS patients. In addition, while ACS is a high thrombotic risk scenario, this risk must be balanced against the risk of bleeding.12 In this respect, European guidelines recommend individualization of DAPT in ACS patients based on the balance of these risks, with less potent and shorter DAPT durations if concerns regarding bleeding risk outweigh those regarding thrombotic risk.2 However, the crucial step in all of this is to be able to identify the right patients. The study by Abu-Assi et al.10 provides reassurance that we can confidently do this using the ARC-HBR criteria in ACS patients.
FUNDINGR. Colleran has received a research grant to the institution from Women As One. No external funding was used for this editorial comment.
CONFLICTS OF INTERESTR. Colleran has received speaker fees from Medtronic. P. Urban is medical director of CERC, Massy, France and consultant to MedAlliance, Nyon, Switzerland and Biosensors, Morges, Switzerland.
.