To the Editor,
The incidence of anomalous origin of a coronary artery from the contralateral coronary cusp is estimated at 0.28% to 1.74%.1 In most cases, these aberrant arteries do not lead to ischemia and are detected as incidental findings on diagnostic coronary angiography. However, they are a recognized potential cause of ischemic symptoms and sudden death, particularly in young athletes with an interarterial course of the anomalous vessel, in which it has been hypothesized that the coronary can become compressed between the aortic and pulmonary arteries. It is also believed that the presence of a coronary anomaly is associated with a greater degree of atherosclerosis and that it occurs more commonly in patients with degenerative aortic valve disease.2
There are few reports of this condition and most are isolated cases; thus, the clinical relevance and management of these anomalies remains to be defined.3
We present the case of two first-degree relatives (father and son) with this condition referred to our center for diagnostic coronary angiography.
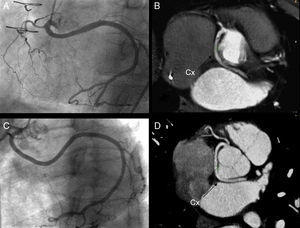
The first patient was an 81-year-old man, who had undergone mechanical mitral and aortic valve implantation to treat rheumatic valvular disease, had a dual-chamber pacemaker, and was hospitalized for progressive dyspnea. Perfusion scintigraphy with dipyridamole technetium showed inferolateral ischemia, and the patient was referred for coronary angiography. The coronary study showed a dominant left circumflex artery (LCx) with anomalous origin in the right coronary cusp and sharing a common ostium with the right coronary artery (RC) (Figure 1).
Figure 1. Coronary angiography (A and C) and 64-channel multislice coronary computed tomography (B and D) showing the retroaortic course of the circumflex artery between the left atrium and aorta in the father (A and B) and son (C and D). Cx, circumflex.
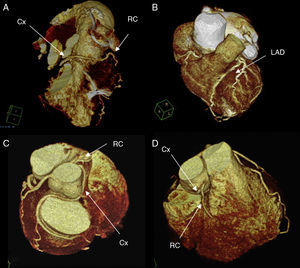
Multislice computed tomography coronary angiography (64 channels) was then performed and the LCx was seen to have a retroaortic course that ran between the left atrium and ascending aorta (Figure 1, Figure 2).
Figure 2. 3-D reconstruction of the heart showing the coronary anatomy of the father (A and B) and son (C and D). Cx, circumflex; LAD, left anterior descending artery; RC, right coronary.
The second patient (son of the first) is a 50-year-old man, smoker, with dyslipidemia, who underwent coronary angiography to investigate chest pain on exertion. The son's coronary anatomy was very similar and could virtually be superimposed on that of his father (Figure 1, Figure 2).
Anomalous origin of the LCx from the right coronary cusp is the second most common coronary artery anomaly (some authors consider a high origin of the RC to be the most common anomaly), and accounts for one-third of all such conditions.4 These vessels are not usually associated with symptoms and are considered the paradigm of “benignity” among these conditions.
A few studies have suggested that these anomalies can show family aggregation. Horan et al. described the case of a father and daughter, both with a single coronary artery5: in the father, the RC originated in the middle LCx, whereas in the daughter, the left coronary artery and RC had a common ostium in the right sinus of Valsalva. Complete surgical revascularization was performed in both patients. Bunce et al. reported the case of two sisters with coronary artery anomalies identified by magnetic resonance imaging. One sister presented anomalous origin of the RC from the sinus of Valsalva, and the other had a single coronary artery originating in the right cusp.6
To our knowledge, this is the first documentation of a coronary artery anomaly in which the anatomy of the vessels is completely similar in two first-degree relatives, with the additional peculiarity that it is a dominant LCx, an unusual circumstance in circumflex arteries of anomalous origin. The probability of this occurring fortuitously in two members of the same family is extremely small; hence, the cases described support the notion that there is a genetic component in this condition.
Treatment of these anomalies is controversial, and there are no specific recommendations for this purpose in clinical guidelines. Barriales et al. have proposed a clinical decision algorithm based on the course of the anomalous vessel, the patient's age, and detection of ischemia.3 In our case, a conservative approach was decided for both patients, with clinical follow-up and periodic examinations to investigate ischemia because of the atypical character of the pain symptoms, the age of the diagnosis, and the absence of an interarterial course of the anomalous vessel.
Screening of first-degree family members in patients with a coronary anomaly may be a reasonable practice, in particular if the condition presents as early sudden death.
Corresponding author: Leireunzue@yahoo.es