Microvascular obstruction (MVO) exerts deleterious effects following acute myocardial infarction (AMI). We investigated coronary angiogenesis induced by coronary serum and the role of hypoxia-inducible factor-1A (HIF-1A) in MVO repair.
MethodsMyocardial infarction was induced in swine by transitory 90-minute coronary occlusion. The pigs were divided into a control group and 4 AMI groups: no reperfusion, 1minute, 1 week and 1 month after reperfusion. Microvascular obstruction and microvessel density were quantified. The proangiogenic effect of coronary serum drawn from coronary sinus on endothelial cells was evaluated using an in vitro tubulogenesis assay. Circulating and myocardial HIF-1A levels and the effect of in vitro blockade of HIF-1A was assessed.
ResultsCompared with control myocardium, microvessel density decreased at 90-minute ischemia, and MVO first occurred at 1minute after reperfusion. Both peaked at 1 week and almost completely resolved at 1 month. Coronary serum exerted a neoangiogenic effect on coronary endothelial cells in vitro, peaking at ischemia and 1minute postreperfusion (32 ± 4 and 41 ± 9 tubes vs control: 3 ± 3 tubes; P < .01). Hypoxia-inducible factor-1A increased in serum during ischemia (5-minute ischemia: 273 ± 52 pg/mL vs control: 148 ± 48 pg/mL; P < .01) being present on microvessels of all AMI groups (no reperfusion: 67% ± 5% vs control: 15% ± 17%; P < .01). In vitro blockade of HIF-1A reduced the angiogenic response induced by serum.
ConclusionsCoronary serum represents a potent neoangiogenic stimulus even before reperfusion; HIF-1A might be crucial. Coronary neoangiogenesis induced by coronary serum can contribute to understanding the pathophysiology of AMI.
Keywords
In acute myocardial infarction (AMI), early reperfusion of the occluded coronary artery is the mandatory step to preserve the viability of the jeopardized myocardial tissue.1–3 However, despite successful restoration of blood flow through the epicardial artery, the microvasculature can remain severely damaged in a considerable number of patients. This phenomenon, known as microvascular obstruction (MVO), consists of a massive loss of small vessels and portends deleterious effects on patient outcome and on left ventricular remodeling.2,4–6 Microvascular obstruction can be considered one of a series of harmful effects of the unwanted ischemia-reperfusion injury paradoxically associated with the restoration of blood flow into the infarcted area. Thus upon reperfusion, coronary serum simultaneously brings about both beneficial and detrimental consequences.1,7,8
Microvascular obstruction is a dynamic process. Experimental and cardiac imaging studies have shown not only a rapid onset after reperfusion, but also a trend toward spontaneous resolution late (in the weeks and months) after AMI.4,6,9 Undoubtedly neoangiogenesis is paramount in the reestablishment of coronary circulation, providing oxygen and nutrients, salvaging ischemic myocardium, contributing to the formation of a solid scar, and avoiding unnecessary left ventricular remodeling.10–12 Various factors have been related to the neoangiogenic process. Among them, hypoxia-inducible factor-1A (HIF-1A) seems to play a crucial role in different scenarios related to neovessel formation.13,14 The timing and mechanisms that regulate neoangiogenesis and MVO repair after AMI have not yet been totally clarified.
We hypothesized that coronary serum promotes neoangiogenesis in coronary endothelial cells and thus contributes to MVO repair from the early phases of AMI. Therefore, as a part of the myriad of beneficial and detrimental effects caused by reperfusion of AMI, coronary serum would participate simultaneously in both the occurrence of MVO and in the initiation of its repair.
Using a highly controlled swine model of AMI and MVO and an in vitro assay of coronary endothelial cells tubulogenesis, the specific objectives of the present study were: a) to define the time course of microvessel loss during the occurrence of MVO and of neovessel formation during MVO repair, b) to evaluate the dynamic neoangiogenic effects of serum derived from coronary veins on coronary endothelial cells from the onset of ischemia until late after reperfusion, and c) to explore the potential mechanistic role of HIF-1A in the angiogenic process post-AMI.
METHODSExperimental ProtocolThis study was approved by the Animal Care and Use Committee of the University of Valencia and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1993), as well as the European (2010/63/EC) and national regulations (RD53/2013). In the experimental study, juvenile domestic female pigs weighing 25 to 30kg were employed.
After engagement of the proximal left anterior descending artery using a transfemoral 6F Amplatz Left 0.75 catheter, an angioplasty balloon (2.5 x 16mm) was placed in the midleft anterior descending and used to induce severe coronary ischemia by transitory (90-minute) balloon inflation.
The complete experimental protocol has been previously validated and can be consulted elsewhere.4
Experimental GroupsThe animals were divided into a control group and 4 independent AMI experimental groups. In the AMI groups, after 90-minute occlusion of the midleft anterior descending by the angioplasty balloon, experiments were categorized as follows: a) no reperfusion, b) 1minute, c) 1 week, and d) 1 month after reperfusion (n = 5 each). The control group (n = 5) was subjected to the same experimental protocol used in the AMI groups, but the angioplasty balloon was not inflated and thus ischemia and infarction were not induced.
Further details regarding materials and methods are specified in the .
RESULTSExperiments were successfully performed in controls (n = 5). Ninety-minute midleft anterior descending coronary artery occlusion was conducted in 25 pigs; 3 of them died during balloon inflation due to refractory ventricular fibrillation and 2 during the reperfusion period. Experiments were successfully completed in the remaining 20 pigs, although electrical ventricular defibrillation was needed during coronary occlusion or immediately after reperfusion in 6 pigs. Patency of the left anterior descending was confirmed in all pigs before sacrifice. Five controls and 20 AMI experiments (no reperfusion, 1minute, 1 week and 1 month after reperfusion; n = 5 each) made up the final study group.
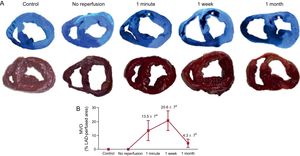
Dynamics of Microvascular ObstructionMacroscopically, controls and the no-reperfusion group displayed a completely intact perfusion. Microvascular obstruction occurred in the myocardial tissue of all reperfused experiments. Microvascular obstruction was first detected in the 1-minute reperfusion group, peaked in the 1-week postreperfusion group, and significantly decreased in the 1-month postreperfusion group (Figure 1). Thus, after the same period of coronary occlusion as the no-reperfusion group (90-minute), MVO took place as soon as 1minute after reperfusion, reached a maximum at 1 week, and tended to spontaneously disappear at 1 month.
Dynamics of MVO in a swine model of reperfused AMI. A: upper panels show macroscopic representative images of heart slices from the control groups and the 4 AMI groups (no reperfusion, 1minute, 1 week and 1 month after reperfusion) stained with thioflavin-S. Asterisks represent the MVO area in the reperfused groups. Lower panels display illustrative images of heart slices stained with 2,3,5-triphenyltetrazolium chloride solution. B: the extent of MVO is quantified as a percentage of the left anterior descending coronary artery-perfused area in the 5 experimental groups. The MVO appeared at early phases post-AMI, peaking after 1-week reperfusion and almost completely resolved 1-month after reperfusion. Data (mean ± standard deviation, n ≥ 5) were analyzed by 1-way ANOVA analysis followed by Bonferroni test. Image analysis was performed by a blinded observer unaware of the experimental group. aP < .01 vs control. bP < .01 vs 1-week reperfusion group. AMI, acute myocardial infarction; LAD, left anterior descending; MVO, microvascular obstruction.
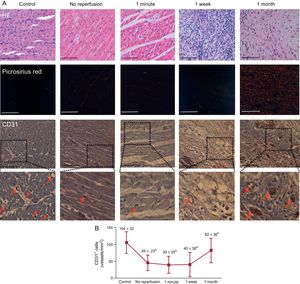
In comparison with controls, a significant reduction in CD31+ microvessel density was detected in the infarcted myocardium samples extracted from the no-reperfusion group after 90-minute ischemia. In the 1-minute and 1-week after reperfusion groups, CD31+ microvessel density remained low and recovered to similar levels nonsignificantly different to controls at 1 month (Figure 2). Thus, microvessel density loss preceded MVO occurrence (prior to reperfusion) and both processes followed a parallel recovery that, in the case of microvessel density, was complete at 1 month. No differences were observed in the number of CD31+ microvessels in myocardial samples isolated from the remote areas in any of the 4 AMI groups in comparison with controls.
Dynamics of microvessel density in a swine model of reperfused myocardial infarction. A: representative images from infarcted tissue isolated from the control group and the 4 myocardial infarction groups (no reperfusion, 1-minute, 1-week and 1-month reperfusion) stained with HE (upper panels), picrosirius red (central panels) and with the specific vascular marker CD31 (lower panels). Red arrows indicate CD31+ vessels. The scale bars indicate 100μm. B: quantification of CD31+ vessels. Images from the infarcted area isolated from the 5 independent groups were analyzed with Image-Pro Plus analysis software. The number of CD31+ vessels was reduced in the infarcted myocardium from the no reperfusion, 1-minute, and 1-week reperfusion groups in comparison with controls while being recovered 1 month after reperfusion. Data (mean ± standard deviation, n ≥ 5) were analyzed by 1-way ANOVA analysis followed by Bonferroni test. Scoring was performed by a blinded observer unaware of the experimental group. aP < .05 vs control. bP < .05 vs 1-week reperfusion group. HE, hematoxylin-eosin.
An in vitro morphogenesis assay using coronary endothelial cells was used to explore the capacity to induce tubulogenesis of coronary serum drawn from coronary veins at different times of ischemia and reperfusion.
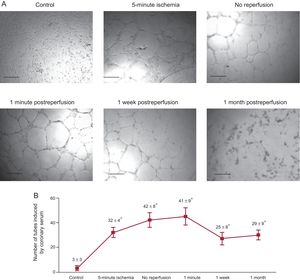
Compared with controls, coronary serum drawn from AMI experiments exerted a potent stimulus to generate tubular-like structures. Of note, the highest angiogenic capacity of coronary serum on endothelial coronary cells occurred at the moment when the microvessel density at the infarcted myocardial area was lowest, namely immediately before and 1minute after reperfusion (Figure 3).
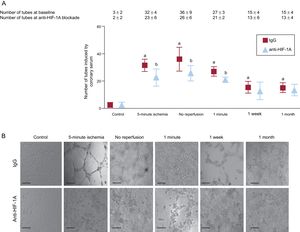
The capacity of coronary serum to induce angiogenesis on coronary endothelial cells. A: representative images of porcine coronary artery endothelial cells in an in vitro differentiation assay induced by coronary serum isolated at control (before balloon inflation) and at different times of the ischemia and reperfusion process: after 5-minute and 90-minute ischemia (immediately before reperfusion) as well as 1minute, 1 week and 1 month after reperfusion following myocardial infarction. Phase contrast micrographs were recorded. The scale bars indicate 500μm. B: quantification of the number of tubes induced by coronary serum. Coronary serum derived from myocardial infarction experiments showed higher angiogenic capacity in comparison with controls, peaking in serum drawn during ischemia and immediately after reperfusion. Data are expressed as mean ± standard deviation of the number of tube-like structures in 5 low-magnification (× 100) fields (n ≥ 5 independent experiments performed in triplicate), and were analyzed by 1-way ANOVA analysis followed by Bonferroni test. Scoring was performed by a blinded observer unaware of the experimental group. *P < .01 vs control.
Thus, in early phases of AMI and simultaneous to a massive loss of microvessel density, coronary serum activates a potent compensatory mechanism aiming to restore perfusion into the infarcted area.
Dynamics of Hypoxia-inducible Factor-1ATo investigate the potential role of HIF-1A in the above-mentioned angiogenic capacity of coronary serum to repair microvascular damage in reperfused AMI, we first evaluated the dynamic changes in HIF-1A levels in coronary serum and, second, we quantified the myocardial expression in the infarcted area at sequential time points of ischemia and reperfusion. Finally, we further investigated ex vivo the functional consequences on angiogenesis of serum HIF-1A blockade.
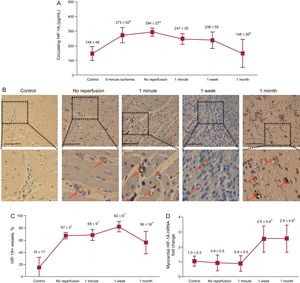
The circulating concentration of HIF-1A in the coronary sinus paralleled the angiogenic effect of coronary serum on coronary endothelial cells: levels were markedly increased after 5-minute and 90-minute ischemia and progressively lowered within the first month after reperfusion (Figure 4A).
Dynamics of HIF-1A following myocardial infarction. A: the circulating levels of HIF-1A in the coronary serum at different times of the ischemia and reperfusion process. The concentration of HIF-1A in the coronary sinus was significantly elevated during ischemia and was progressively lowered during the first month after reperfusion. Data (mean ± standard deviation, n ≥ 5) were analyzed by 1-way ANOVA analysis followed by Bonferroni test. aP < .01 vs control. bP < .05 vs no reperfusion. B: immunohistochemistry analysis of HIF-1A expression in the infarcted myocardium at different times of the ischemia and reperfusion process. Representative images of the infarcted area from the control group and the 4 myocardial infarction groups (no reperfusion, 1-minute, 1-week and 1-month reperfusion) are shown. Arrows indicate HIF-1A+ vessels. The scale bars indicate 100μm. C: quantification of the presence of HIF-1A in the infarcted myocardium. Images from the infarcted area isolated from the 5 independent groups were analyzed with Image-Pro Plus analysis software. The percentage of HIF+ vessels was significantly increased in the infarcted myocardium from all AMI groups. Data (mean ± standard deviation, n ≥ 5) were analyzed by 1-way ANOVA analysis followed by Bonferroni test. Scoring was performed by a blinded observer unaware of the experimental group. *P < .01 vs control. D: expression of HIF-1A in the infarcted myocardium at different times of the ischemia and reperfusion process. HIF-1A mRNA expression was upregulated in the infarcted myocardium 1 week and 1 month after reperfusion in comparison with controls. Data (mean ± standard deviation, n ≥ 5 independent experiments performed in triplicate) were analyzed by 1-way ANOVA analysis followed by Bonferroni test. *P < .05 vs control. AMI, acute myocardial infarction; HIF-1A, hypoxia-inducible factor-1A.
A weak constitutive gene expression of HIF-1A occurred in controls and in the infarcted area from the no-reperfusion and 1-minute after reperfusion groups. Of interest, a marked upregulation of HIF-1A was detected in the myocardial samples obtained from the infarcted area of the 1-week and 1-month after reperfusion groups (Figure 4D). However, when analyzing the presence of HIF-1A on the infarcted myocardium, we observed that HIF-1A staining was significantly augmented on microvascular endothelial cells from the infarcted area of all AMI groups (Figure 4B and Figure 4C). Neither the mRNA expression nor the immunohistochemistry studies showed an increase in HIF-1A expression in myocardial samples isolated from the remote areas in any of the 4 AMI groups in comparison with controls.
In summary, circulating levels of HIF-1A rise soon after ischemia and reperfusion and parallel the in vitro proangiogenic effects exerted by coronary serum. Nevertheless, a certain delay occurs until myocardial up-regulation takes place.
Potential Mechanistic Role of Hypoxia-inducible Factor-1A on the Proangiogenic Capacity of Coronary Serum on Coronary Endothelial CellsTo explore the potential mechanistic role of HIF-1A in the proangiogenic effect of coronary serum on coronary endothelial cells described above, we investigated ex vivo the consequences on angiogenesis of functional HIF-1A serum neutralization using Matrigel, as previously described.15 We observed that HIF-1A blockade by a specific monoclonal antibody negated the capacity of coronary serum to generate new tubular structures at the same time points that, under baseline conditions, coronary serum exerted a potent proangiogenic effect (Figure 5).
Functional implication of HIF-1A in the proangiogenic capacity of coronary serum on coronary artery endothelial cells. A: quantification of the effect of HIF-1A neutralization in a number of tube-like structures induced by coronary serum. Porcine coronary endothelial cells were incubated with serum isolated at control (before balloon inflation), after 5-minute and 90-minute ischemia (immediately before reperfusion). Some cells were incubated in the presence of a mouse monoclonal antiporcine HIF-1A antibody (10μg/mL) or irrelevant isotype and concentration-matched IgG. Phase contrast micrographs were recorded and the number of tube-like structures were counted. Data are expressed as mean ± standard deviation of the number of tube-like structures in 5 low-magnification (× 100) fields (n ≥ 5 independent experiments performed in triplicate), and were analyzed by 1-way ANOVA analysis followed by Bonferroni test. Scoring was performed by a blinded observer unaware of the experimental group. aP < .01 vs control. bP < .05 vs irrelevant isotype-matched immunoglobulin. B: representative images of endothelial cell differentiation assay on Matrigel induced by coronary serum. The scale bars indicate 200μm. HIF-1A, hypoxia-inducible factor-1A; IgG, immunoglobulin G.
Thus, this in vitro analysis suggests that HIF-1A plays a relevant role in the proangiogenic capacity of coronary serum in situations of severe coronary ischemia and reperfusion.
DISCUSSIONThe main contribution of the present study is that in the context of AMI, coronary serum has the ability to promote in vitro coronary neoangiogenesis from the onset of ischemia and that this process occurs in parallel with the restoration of microvessel density and MVO repair in the infarcted area. Upregulation of HIF-1A seems to be crucial for the beneficial effects of coronary serum on coronary microcirculation.
Dynamics of Microvascular Obstruction and Microvessel DensityDespite timely and complete restoration of infarct vessel patency, MVO occurs in a significant number of patients and exerts a strong negative impact after AMI in terms of prognosis and left ventricular remodeling.5,6,9
The timing, as well as the mechanisms underlying MVO restoration, are far from totally understood. A better knowledge of this process is of utmost importance in advancing our understanding of the pathophysiology of AMI and for future exploration of new therapeutic opportunities beyond reperfusion of the epicardial artery.
We undertook experiments in a previously validated porcine model of reperfused AMI and MVO.4 Macroscopically, our study confirmed the dynamic course of MVO: it was detected immediately after reperfusion, peaked at 1 week, and almost completely vanished at 1 month.4
Lack of microvascular integrity in samples obtained from infarcted areas has been previously reported7 but its association with the dynamics of MVO in a highly controlled model such as ours has not been analyzed so far.
Based on the quantification of CD31+ cells, a severe loss of microvessel density in the infarcted area preceded the occurrence of macroscopic MVO in myocardial samples obtained during severe (90-minute) ischemia without reperfusion. Microvascular damage persisted during the first week and recovered to control levels 1 month after reperfusion.
Thus, coronary flow plays a multifaceted role in the pathophysiology of AMI and MVO at successive time points. First, a sudden interruption of coronary perfusion is decisive, not only at the onset of myocardial necrosis but, according to our results, also in triggering microvascular damage.4 Then, restoration of blood flow into the infarcted area, though mandatory for myocardial salvage, has been widely demonstrated to potentially enhance infarct size and MVO through a myriad of mechanisms related to ischemia-reperfusion injury.1,2,6 In fact, in our experiments, with the same time of ischemia, significant macroscopic MVO occurred as soon as 1minute after reperfusion but not in the no-reperfusion group (Figure 6A).
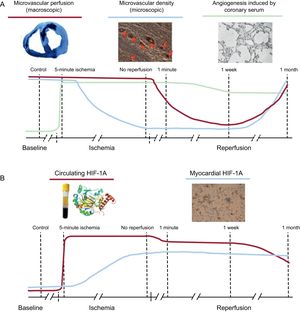
Dynamics of microvascular damage, coronary serum-induced angiogenesis and levels of HIF-1A in the ischemia and reperfusion process following myocardial infarction. A: in reperfused myocardial infarction, microvessel density loss preceded microvascular obstruction occurrence (prior to reperfusion), after which both phenomena recovered in parallel with almost complete reestablishment 1 month after reperfusion. In addition, coronary serum exerts a potent compensatory mechanism soon after ischemia aiming to restore the perfusion into the infarcted region. B: the dynamic of circulating HIF-1A increases dramatically during ischemia and reperfusion, which mirrors the proangiogenic effect of coronary serum on coronary endothelial cells. A certain delay occurs before myocardial upregulation takes place 1 week postreperfusion. HIF-1A, hypoxia-inducible factor-1A.
We showed that recovery of the microvasculature underlies the resolution of MVO 1 month after reperfusion. The next step in the present study addressed whether coronary serum was also decisive in stimulating the coronary neoangiogenic process that mediates MVO repair.
Coronary Serum and Angiogenesis Following Myocardial InfarctionAngiogenesis represents the development of newly formed microvessels from pre-existing capillaries in response to different signals such as hypoxia, growth factors, or oxidative stress.10,11 The crucial reparative role of this process post-AMI has been extensively demonstrated.10,12 Nevertheless, to our knowledge, the potential for the coronary serum to induce neoangiogenesis and consequently restore MVO, as well as the timing of this process, have not been reported so far. To fulfill these objectives, we obtained coronary serum at sequential AMI time points. Then, in a functional morphogenesis assay, we tested the capacity of the serum to stimulate the formation of new vascular structures in coronary endothelial cells. We observed that, in comparison with controls, coronary serum derived from AMI experiments exerted a potent neoangiogenic effect. Interestingly, this capacity peaked in serum drawn during ischemia and immediately after reperfusion, and remained higher than in controls during the first month after AMI. This suggests that even before reperfusion, serological mechanisms are underway aimed at restoring the microvascular damage that accompanies AMI. The observed augmentation of neovessels in the infarcted area and the spontaneous resolution of MVO 1 month after reperfusion are probably, at least in part, a consequence of the neoangiogenic effect exerted by coronary serum from the onset of ischemia (Figure 6A).
Undoubtedly, the observed angiogenic stimulus exerted by coronary serum is a multifactorial phenomenon triggered by a wide number of mediators. To further advance knowledge of this process, we focused on the dynamics and potential mechanistic role of the transcription regulatory factor HIF-1A.
Dynamics of Hypoxia-inducible Factor-1A Following Myocardial Infarction. Role in AngiogenesisHypoxia-inducible factor-1A increases in response to physiological and pathological hypoxia. This protein is involved in the activation of anaerobic glycolysis and target genes related to neoangiogenesis and survival of viable tissue surrounding hypoxic areas.10,16,17 In AMI, its implication was assessed in human biopsies from infarcted hearts18,19 and in experimental AMI models.13,14 Moreover, HIF-1A has also been demonstrated to play a pivotal role in pre- and postconditioning.20–22 These processes consist of brief episodes of ischemia either before coronary occlusion (the former) or immediately after reperfusion (the latter) and have been related to beneficial effects in terms of myocardial salvage. Release of HIF-1A occurs in both scenarios.21,22
In our study, the time course of circulating HIF-1A levels displayed dramatic fluctuations, peaking during ischemia and soon after reperfusion. This dynamic mirrors the angiogenic effect of coronary serum on in vitro coronary endothelial cells (Figure 6).
Using anti-HIF-1A neutralizing antibodies to block the angiogenic effect of coronary serum in vitro confirmed the functional role of this factor in post-AMI coronary neoangiogenesis and MVO repair.
Hypoxia-inducible factor-1A expression has been correlated with new capillary growth in a variety of scenarios.10,16,17 Our study illustrates in a highly controlled manner the dynamics and timing of microvascular repair stimulated by coronary serum and particularly by HIF-1A in the setting of AMI with MVO. The presence of HIF-1A on microvascular endothelial cells from the infarcted region of all AMI groups was detected in parallel to the almost immediate rise of HIF-1A in serum after the onset of ischemia and its increased capacity to generate in vitro vascular structures. However, HIF-1A mRNA expression was only upregulated 1 week and 1 month after reperfusion. This might indicate that, although HIF-1A is already promoting angiogenesis on coronary endothelium even before reperfusion, a certain delay occurs in the myocardial expression of this factor. In our study, myocardial presence of HIF-1A peaked before reperfusion and thereafter was followed by microvessel density recovery and MVO restoration at 1 month after AMI (Figure 6B).
Contrary to the notion of irreversibility of infarct size, by using imaging techniques in patients and highly controlled experimental models in animals, our group and others have demonstrated the tendency toward spontaneous resolution of macroscopic MVO during the weeks and months following reperfused AMI.4,6,9 Delayed MVO repair as derived from cardiac magnetic resonance has been suggested to portend severe structural left ventricular consequences in post-AMI patients.23 Our results show that coronary serum and particularly the HIF-1A pathway plays a pivotal role in microvascular regeneration after AMI. Further studies are needed to investigate whether therapeutic maneuvers addressed to accelerate this natural process may represent a realistic option beyond coronary reperfusion.
LimitationsThe course of microvascular damage and repair, as well as the proangiogenic effect exerted by coronary serum and HIF-1A on coronary endothelial cells, have been demonstrated in a highly controlled scenario using experimental and in vitro models.
Our AMI model was performed through acute occlusion of a normal coronary artery, which is not the same as an acute occlusion in an acute myocardial clinical setting, where erosion and/or plaque rupture are present. These results need confirmation in patients with spontaneous AMI.
The therapeutic implications of our findings are far beyond the scope of the present study and have not been addressed.
CONCLUSIONSIn the setting of AMI, microvascular damage and the compensatory mechanism for its repair begin simultaneously even before coronary reperfusion. Triggered by myocardial ischemia, coronary serum and particularly HIF-1A exert potent neoangiogenic stimulation that starts soon after coronary occlusion and ends with an almost complete macro- and microscopic reconstruction of microcirculation in the infarcted area 1 month later. Coronary serum-induced neoangiogenesis in coronary endothelial cells represents a concept that could contribute to the understanding of the pathophysiology of AMI and may be used to develop new therapeutic opportunities beyond coronary reperfusion.
FUNDINGThe present study was supported by Instituto de Salud Carlos III and European Regional Development Fund (PIE15/00013, PI14/00271, CPII13/00025, PI12/01271, PI15/00082, and CB16/11/00486 grants) and Generalitat Valenciana (PROMETEO/2013/007 grant).
CONFLICTS OF INTERESTNone declared.
- –
In the AMI scenario, MVO consists of a massive loss of microvessels after reperfusion of the occluded epicardial artery, provoking deleterious effects on patient outcome.
- –
Although experimental and cardiac imaging studies have widely demonstrated spontaneous resolution of MVO late after AMI, the implicated mechanisms are far from completely understood.
- –
Angiogenesis is essential to re-establish coronary circulation and to avoid unnecessary left ventricular remodeling. In particular, the proangiogenic factor HIF-1A has been described in multiple physiological and pathological situations of hypoxia.
- –
Microvascular damage and a compensatory mechanism for its repair begin simultaneously, even before coronary reperfusion.
- –
Coronary serum, particularly HIF-1A, has the capability to promote in vitro angiogenesis from the onset of ischemia, probably participating in the augmentation of neovessels in the infarcted area and the resolution of MVO 1 month after reperfusion.