To analyze the cost-effectiveness ratio and budget impact of treatment with evolocumab (PCSK9 inhibitor) for patients in secondary prevention in the Spanish National Health System.
MethodsA budget impact analysis, decision tree and Markov models were designed under the public health system perspective, based on the only study with morbidity and mortality data (FOURIER). The alternatives compared were evolocumab vs statins, and dual therapy with ezetimibe in 5% of the population. The measure of effectiveness used was the number of cardiovascular events avoided. Univariate and probabilistic sensitivity analyses were performed.
ResultsThe average annual cost of patients receiving evolocumab was 11 134.78€ and 393.83€ for standard treatment (statins plus ezetimibe). The incremental cost-effectiveness ratio was > 600 000 € per avoided cardiovascular event for both assessed outcomes (first: cardiovascular death, myocardial infarction, stroke, and hospitalization due to unstable angina or coronary revascularization; second: includes the first 3 events). To perform the 10-year Markov model, the average cost of standard treatment was 13 948.45€ vs 471 417.37€ with evolocumab. Treatment with evolocumab for patients with familial hypercholesterolemia would cost between 3 and 6.1 million euros, assuming a difference of 2.5 and 5.1 million euros with the standard treatment (2017). This difference would be between 204.3 and 1364.7 million euros (2021) for those with nonfamiliar hypercholesterolemia (secondary prevention).
ConclusionsTreatment with evolocumab is associated with a lower frequency of cardiovascular events, but is inefficient for patients suitable to receive this drug in the Spanish National Health System.
Keywords
Cardiovascular disease is associated with a high incidence of morbidity and mortality.1 A major risk factor for cardiovascular events is an individual's atherogenic lipid profile, particularly a high concentration of low-density lipoprotein cholesterol (LDL-C). The standard cholesterol-lowering treatment is statin therapy, but in patients with statin intolerance or a contraindication, large reductions in LDL-C can be achieved with inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9).2
A Cochrane review (2017) including 20 clinical trials and a total of 67 237 participants (median age 61 years; range, 52- 64 years) found that PCSK9 inhibitors reduced LDL-C by 53.86% vs placebo (95% confidence interval [95%CI], 58.64-49.08; 4782 participants), by 30.20% vs ezetimibe (95%CI, 34.18-26.23; 823 participants), and by 39.20% vs statins plus ezetimibe (95%CI, 56.15-22.26; 5376 participants).2 The studies included in the review had a short-term follow-up (maximum 26 months), and although the studies did not include reductions in cardiovascular events as a main endpoint, the review revealed a modest protective effect (< 1%) with a high level of uncertainty. In March 2017, the first study was published examining cardiovascular morbidity and mortality with the PCSK9 inhibitor evolocumab after a 26-month follow-up.3 Another large morbidity and mortality study is currently evaluating the PCSK9 inhibitor alirocumab.4
The European Medicines Agency has approved alirocumab and evolocumab for familial hypercholesterolemia or secondary prevention in dyslipidemia patients in whom statins provide insufficient cholesterol control, due either to refractoriness or to intolerance. These drugs have been commercialized at a much higher price than other cholesterol-lowering drugs, despite the lack of availability of appropriate morbidity and mortality studies.5,6 Against a background of limited resources, it is important to increase the efficiency of available treatments. With this goal in mind, the aim of this study was to estimate the cost-effectiveness ratio and budget impact of evolocumab therapy in the Spanish National Health System.
METHODSWe carried out 2 types of economic evaluation: a decision tree (time horizon, 26 months) and a 10-year simulation using a Markov model based on survival-curve analysis.7 Both analyses used data from the FOURIER trial,3 and the effectiveness measure was averted cardiovascular events.
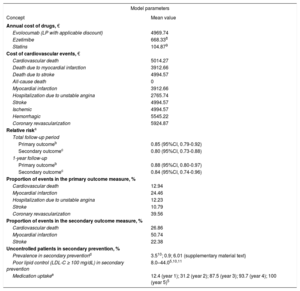
Treatment AlternativesIn the FOURIER trial, evolocumab was administered on a background of standard statin therapy according to the patient baseline characteristics.3 Patients received either 420mg every 4 weeks or 140mg every 2 weeks; however, we based cost calculations on the biweekly pattern, as this is the regimen presented in the technical data sheet; in the absence of disaggregated data, both dose regimens were assumed to have similar efficacy. In the study, approximately 70% of patients received high-intensity statin therapy and the other 30% moderate-intensity statin therapy. In both situations, approximately 5% of the patients received concomitant ezetimibe therapy. The cost and effectiveness data used are summarized in Table 1.3,5,8–11
Prevalence, Efficacy, and Costs in the Budget Impact and Economic Evaluation Analyses
| Model parameters | |
|---|---|
| Concept | Mean value |
| Annual cost of drugs, € | |
| Evolocumab (LP with applicable discount) | 4969.74 |
| Ezetimibe | 668.338 |
| Statins | 104.879 |
| Cost of cardiovascular events, € | |
| Cardiovascular death | 5014.27 |
| Death due to myocardial infarction | 3912.66 |
| Death due to stroke | 4994.57 |
| All-cause death | 0 |
| Myocardial infarction | 3912.66 |
| Hospitalization due to unstable angina | 2765.74 |
| Stroke | 4994.57 |
| Ischemic | 4994.57 |
| Hemorrhagic | 5545.22 |
| Coronary revascularization | 5924.87 |
| Relative riska | |
| Total follow-up period | |
| Primary outcomeb | 0.85 (95%CI, 0.79-0.92) |
| Secondary outcomec | 0.80 (95%CI, 0.73-0.88) |
| 1-year follow-up | |
| Primary outcomeb | 0.88 (95%CI, 0.80-0.97) |
| Secondary outcomec | 0.84 (95%CI, 0.74-0.96) |
| Proportion of events in the primary outcome measure, % | |
| Cardiovascular death | 12.94 |
| Myocardial infarction | 24.46 |
| Hospitalization due to unstable angina | 12.23 |
| Stroke | 10.79 |
| Coronary revascularization | 39.56 |
| Proportion of events in the secondary outcome measure, % | |
| Cardiovascular death | 26.86 |
| Myocardial infarction | 50.74 |
| Stroke | 22.38 |
| Uncontrolled patients in secondary prevention, % | |
| Prevalence in secondary preventiond | 3.510; 0.9; 6.01 () |
| Poor lipid control (LDL-C ≥ 100 mg/dL) in secondary prevention | 8.0–44.05,10,11 |
| Medication uptakee | 12.4 (year 1); 31.2 (year 2); 87.5 (year 3); 93.7 (year 4); 100 (year 5)5 |
LDL-C, low-density lipoprotein cholesterol; LP, laboratory price; 95%CI, 95% confidence interval.
Data from the FOURIER study,3 including patients in secondary prevention with statin intolerance or an insufficient treatment response.
The preferred outcome measure of a cost-effectiveness analysis is quality-adjusted life-years gained. However, the focus of the current analysis was the effectiveness at averting cardiovascular events and the associated treatment costs. The measures used here are the efficacy endpoints considered in the FOURIER trial3: a) primary: the composite of cardiovascular death, myocardial infarction, stroke, hospitalization due to unstable angina, or coronary revascularization, and b) secondary: the composite of cardiovascular death, myocardial infarction, or stroke.
Cost EstimationCosts were estimated from the perspective of the Spanish National Health System, and therefore indirect costs due to productivity loss were excluded. The costs of events identified in the FOURIER trial3 were estimated according to the Spanish Ministry of Health Diagnostic Related Groups patient classification system, with severity gauged from event incidence in the public health system in Andalusia. The cost of evolocumab therapy was estimated from the unit cost cited in the Spanish prescription medicines registry (Nomenclátor) in September 2017. The cost of ezetimibe therapy was obtained from the Spanish College of Pharmacists online BOT resource,8 and the cost of statin therapy was obtained from Villa et al.9
Budget Impact AnalysisThe Spanish Medicines Agency guidelines (Informe de Posicionamiento Terapéutico) for evolocumab define treatment-eligible patients as those whose hypercholesterolemia is not controlled by standard therapy (LDL-C not brought to ≤ 100mg/dL). This criterion applies whether failed statin therapy is due to nonresponsiveness to the maximum dose, intolerance, or a contraindication and applies equally to patients with homozygotic or heterozygotic familial hypercholesterolemia and to those with established cardiovascular disease.13
The budget impact analysis14 for patients in secondary prevention10,11 was based on prevalence data and projection over a 5-year time horizon (2017-2021) (Table 1). The treatment-eligible population was estimated from data in various registries and reports, and when necessary was extrapolated to the national total.15–21 Moreover, the assumption was made that 14% of individuals would not achieve LDL-C reductions to < 100mg/dL with standard statin therapy, due either to ineffectiveness of the maximum tolerated dose or to intolerance. The sensitivity analysis examined secondary prevention prevalence rates of 0.9% and 6.01% (). For both alternatives, the analysis assumed secondary prevention with evolocumab + statins vs statins or vs statins + ezetimibe (5% of patients) and the event probabilities associated with these treatment alternatives. The populations (> 18 years) for each of the years analyzed were obtained from Spanish National Institute of Statistics population estimates (2016).22
Economic EvaluationDecision trees provide a simplified representation of the choice of the most cost-effective alternative. The results are expressed as the cost per averted cardiovascular event with evolocumab (evolovumab + statins) vs the cost per averted event with standard therapy (statins and statins + ezetimibe), calculated as the incremental cost-effectiveness ratio (ICER) = (cost of alternative B – cost of alternative A) / (efficacy B – efficacy A).
The uncertainty level was evaluated with a univariate sensitivity analysis for a 46% reduction in the price of evolocumab.23 In addition, a probabilistic sensitivity analysis was carried out in Excel with the frequency of the primary and secondary outcomes (beta probability distribution), allowing evaluation of the parametric uncertainty of the probabilities through 1000 simulations.
Markov Model: Effectiveness and AssumptionsDisease progression was simulated using 2 mutually exclusive health states. All patients were entered in the model in the progression-free state and either remained in this state or transitioned to the new event state, depending on the transition probabilities. A general proportion of each type of event was assumed in the final computation. In this type of model, transitions between states take place in discrete periods called cycles; the current model used a cycle duration of 1 month and a time horizon of 120 months. Following the recommendations of López Bastida et al.,24 the sensitivity analyses were carried out with discounting at rates of 3.5% and 6%. (The discounting rate refers to the fact that costs and outcomes may occur at different times, whereas the comparison is made at a single moment; the discounting rate is thus a rate of adjustment for the passage of time.)
Monthly probabilities were calculated by survival curve modeling. Data points were obtained from digitized survival curves and were used together with the published aggregated survival data to recreate the Kaplan-Meier curves using the algorithm of Guyot et al.25 The generated data were compared with the orginal data by calculating the Cox regression hazard ratio (HR). Different parametric distributions were analyzed (exponential, lognormal, Weibull, gamma, gamma-generalized, and log-logistic), and we selected the one giving the best fit to Akaike and Bayesian information criteria (). Finally, the the Simpson rule was used to calculate the area under the curve (AUC), which represents the mean time that patients were free of events (); AUC0→36months and AUC0→120months were calculated for both treatment branches and for both outcomes analyzed. All calculations were made using the Flexsurv package in the R statistical program.26
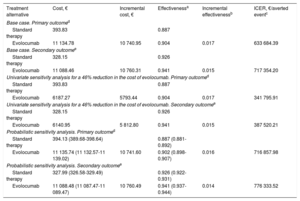
RESULTSCost-Effectiveness Analysis: Decision TreeThe results at 26 months showed that patients treated with evolocumab had event rates for the primary and secondary efficacy endpoints of 9.8% and 5.9%, respectively, compared with 11.3% and 7.4% for patients receiving standard therapy. The mean base-case per-patient cost of evolocumab therapy was €11 134.78 for the primary outcome and €11 088.46 for the secondary outcome; the corresponding costs for patients receiving standard therapy were €393.83 and €328.15, respectively. In the base-case analysis, the ICER (the additional cost per averted cardiovascular event or death) was €633 684.39 for the primary outcome and €717 354.20 for the secondary outcome. Sensitivity analysis for a 46% reduction in the price of evolocumab yielded corresponding ICER values of €341 795.91 and €387 520.21. In the probabilistic analysis, the ICER was €716 857.98 for the primary outcome and €776 333.52 for the secondary outcome (Table 2).
Incremental Cost-effectiveness of Evolocumab Versus Standard Therapy: Base-Case and Sensitivity Analysis Over a 26-month Time Horizon
| Treatment alternative | Cost, € | Incremental cost, € | Effectivenessa | Incremental effectivenessb | ICER, €/averted eventc |
|---|---|---|---|---|---|
| Base case. Primary outcomed | |||||
| Standard therapy | 393.83 | 0.887 | |||
| Evolocumab | 11 134.78 | 10 740.95 | 0.904 | 0.017 | 633 684.39 |
| Base case. Secondary outcomee | |||||
| Standard therapy | 328.15 | 0.926 | |||
| Evolocumab | 11 088.46 | 10 760.31 | 0.941 | 0.015 | 717 354.20 |
| Univariate sensitivity analysis for a 46% reduction in the cost of evolocumab. Primary outcomed | |||||
| Standard therapy | 393.83 | 0.887 | |||
| Evolocumab | 6187.27 | 5793.44 | 0.904 | 0.017 | 341 795.91 |
| Univariate sensitivity analysis for a 46% reduction in the cost of evolocumab. Secondary outcomee | |||||
| Standard therapy | 328.15 | 0.926 | |||
| Evolocumab | 6140.95 | 5 812.80 | 0.941 | 0.015 | 387 520.21 |
| Probabilistic sensitivity analysis. Primary outcomed | |||||
| Standard therapy | 394.13 (389.68-398.64) | 0.887 (0.881-0.892) | |||
| Evolocumab | 11 135.74 (11 132.57-11 139.02) | 10 741.60 | 0.902 (0.898-0.907) | 0.016 | 716 857.98 |
| Probabilistic sensitivity analysis. Secondary outcomee | |||||
| Standard therapy | 327.99 (326.58-329.49) | 0.926 (0.922-0.931) | |||
| Evolocumab | 11 088.48 (11 087.47-11 089.47) | 10 760.49 | 0.941 (0.937-0.944) | 0.014 | 776 333.52 |
ICER, incremental cost-effectiveness ratio.
For the probabilistic sensitivity analysis, hundreds of simulations were conducted with random variation of parameters according to their probability distribution.
For the primary outcome measure, the HR obtained in the cohort simulation was similar to that reported in the trial (HR = 0.85; 95%CI, 0.79-0.91). In the survival curve modeling ( and ), the lognormal distribution gave the best fit for both treatment branches (). The HR obtained in the cohort simulation for the second outcome was also similar to the trial value (HR = 0.80; 95%CI, 0.73-0.87). Once the survival curves were defined, the 120-month cumulative incidence was calculated. For the primary outcome measure, the cumulative incidences for the evolocumab and standard therapy groups were 0.263 (95%CI, 0.251-0.279) and 0.313 (95%CI, 0.298-0.330), respectively; for the secondary outcome, the values were 0.168 (95%CI, 0.156-0.182) and 0.216 (95%CI, 0.202-0.232).
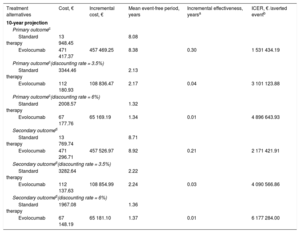
The Markov model analysis for the 10-year horizon is shown in Table 3. For the primary outcome, the projected mean cost of standard therapy with no discounting rate applied was €13 948.45, contrasting with €471 417.37 for evolocumab. This translates into a 10-year ICER of €1 531 434.19, which represents the projected cost of averting 1 additional cardiovascular event upon switching from standard therapy to evolocumab. Application of the 3.5% and 6% discounting rates produced ICER values of €3 101 123.88 and €4 896 643.93, respectively. For the secondary outcome, the switch from standard therapy to evolocumab incurred an additional cost of €2 171 421.91 for each averted event with no discounting. Applying the 3.5% and 6% discounting rates increased this cost to €4 090 566.86 and €6 177 284.00, respectively.
Markov Model of Evolocumab Versus Standard Therapy, With or Without a Discounting Rate Over a 10-year Time Horizon
| Treatment alternatives | Cost, € | Incremental cost, € | Mean event-free period, years | Incremental effectiveness, yearsa | ICER, € /averted eventb |
|---|---|---|---|---|---|
| 10-year projection | |||||
| Primary outcomec | |||||
| Standard therapy | 13 948.45 | 8.08 | |||
| Evolocumab | 471 417.37 | 457 469.25 | 8.38 | 0.30 | 1 531 434.19 |
| Primary outcomec(discounting rate = 3.5%) | |||||
| Standard therapy | 3344.46 | 2.13 | |||
| Evolocumab | 112 180.93 | 108 836.47 | 2.17 | 0.04 | 3 101 123.88 |
| Primary outcomec(discounting rate = 6%) | |||||
| Standard therapy | 2008.57 | 1.32 | |||
| Evolocumab | 67 177.76 | 65 169.19 | 1.34 | 0.01 | 4 896 643.93 |
| Secondary outcomed | |||||
| Standard therapy | 13 769.74 | 8.71 | |||
| Evolocumab | 471 296.71 | 457 526.97 | 8.92 | 0.21 | 2 171 421.91 |
| Secondary outcomed(discounting rate = 3.5%) | |||||
| Standard therapy | 3282.64 | 2.22 | |||
| Evolocumab | 112 137.63 | 108 854.99 | 2.24 | 0.03 | 4 090 566.86 |
| Secondary outcomed(discounting rate = 6%) | |||||
| Standard therapy | 1967.08 | 1.36 | |||
| Evolocumab | 67 148.19 | 65 181.10 | 1.37 | 0.01 | 6 177 284.00 |
ICER, incremental cost-effectiveness ratio.
Annual discounting rate for costs and outcomes to adjust for the passage of time.
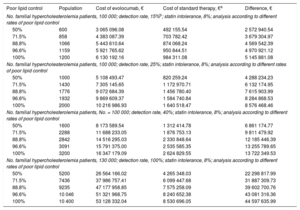
The budget impact was analyzed by comparing evolocumab therapy (evolocumab + statins) with standard therapy (statins + ezetimibe) for a range of scenarios in 2017. The first scenario considered a population of 100 000 patients with familial hypercholesterolemia, a detection rate of 15%, and rates of poor lipid control between 50% and 100%. In these scenarios, the cost of evolocumab therapy would range between €3 million and €6 million, corresponding to €2.5 million and €5.1 million more than the cost of standard therapy. In the other scenarios examined, the cost difference between evolocumab and standard therapy ranged from €4.2 million to €44.5 million (Table 4).
Annual Treatment Costs For Familial Hypercholesterolemia Patients in Different Scenarios (2017)
| Poor lipid control | Population | Cost of evolocumab, € | Cost of standard therapy, €a | Difference, € |
|---|---|---|---|---|
| No. familial hypercholesterolemia patients, 100 000; detection rate, 15%b; statin intolerance, 8%; analysis according to different rates of poor lipid control | ||||
| 50% | 600 | 3 065 096.08 | 492 155.54 | 2 572 940.54 |
| 71.5% | 858 | 4 383 087.39 | 703 782.42 | 3 679 304.97 |
| 88.8% | 1066 | 5 443 610.64 | 874 068.24 | 4 569 542.39 |
| 96.6% | 1159 | 5 921 765.62 | 950 844.51 | 4 970 921.12 |
| 100% | 1200 | 6 130 192.16 | 984 311.08 | 5 145 881.08 |
| No. familial hypercholesterolemia patients, 100 000; detection rate, 25%; statin intolerance, 8%; analysis according to different rates of poor lipid control | ||||
| 50% | 1000 | 5 108 493.47 | 820 259.24 | 4 288 234.23 |
| 71.5% | 1430 | 7 305 145.65 | 1 172 970.71 | 6 132 174.95 |
| 88.8% | 1776 | 9 072 684.39 | 1 456 780.40 | 7 615 903.99 |
| 96.6% | 1932 | 9 869 609.37 | 1 584 740.84 | 8 284 868.53 |
| 100% | 2000 | 10 216 986.93 | 1 640 518.47 | 8 576 468.46 |
| No. familial hypercholesterolemia patients, No. = 100 000; detection rate, 40%; statin intolerance, 8%; analysis according to different rates of poor lipid control | ||||
| 50% | 1600 | 8 173 589.54 | 1 312 414.78 | 6 861 174.77 |
| 71.5% | 2288 | 11 688 233.05 | 1 876 753.13 | 9 811 479.92 |
| 88.8% | 2842 | 14 516 295.03 | 2 330 848.64 | 12 185 446.39 |
| 96.6% | 3091 | 15 791 375.00 | 2 535 585.35 | 13 255 789.65 |
| 100% | 3200 | 16 347 179.09 | 2 624 829.55 | 13 722 349.53 |
| No. familial hypercholesterolemia patients, 130 000; detection rate, 100%; statin intolerance, 8%; analysis according to different rates of poor lipid control | ||||
| 50% | 5200 | 26 564 166.02 | 4 265 348.03 | 22 298 817.99 |
| 71.5% | 7436 | 37 986 757.41 | 6 099 447.68 | 31 887 309.73 |
| 88.8% | 9235 | 47 177 958.85 | 7 575 258.09 | 39 602 700.76 |
| 96.6% | 10 046 | 51 321 968.75 | 8 240 652.38 | 43 081 316.36 |
| 100% | 10 400 | 53 128 332.04 | 8 530 696.05 | 44 597 635.99 |
The model assumes that 8% of familial hypercholesterolemia patients (homozygotic or heterozygotic) are statin intolerant. Possible rates of poor lipid control are taken from the literature.10,11,14 Estimates are presented for different levels of uncertainty.
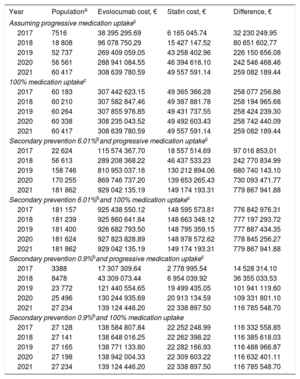
According to the assumptions considered, the budget impact analysis predicted that 7516 treatment-eligible patients with uncontrolled hypercholesterolemia would be receiving evolocumab therapy in 2017. By 2021, this number would be as high as 60 417 patients, depending on the rate of uptake. For 2021, and depending on the assumpations made, the projected cost difference between evolocumab and standard therapy would range from €116 785 548.70 to €779 867 941.88 (Table 5).
Treatment Costs for Treatment-eligible Secondary Prevention Patients With Uncontrolled Hypercholesterolemia
| Year | Populationa | Evolocumab cost, € | Statin cost, € | Difference, € |
|---|---|---|---|---|
| Assuming progressive medication uptakec | ||||
| 2017 | 7516 | 38 395 295.69 | 6 165 045.74 | 32 230 249.95 |
| 2018 | 18 808 | 96 078 750.29 | 15 427 147.52 | 80 651 602.77 |
| 2019 | 52 737 | 269 409 059.05 | 43 258 402.96 | 226 150 656.08 |
| 2020 | 56 561 | 288 941 084.55 | 46 394 616.10 | 242 546 468.46 |
| 2021 | 60 417 | 308 639 780.59 | 49 557 591.14 | 259 082 189.44 |
| 100% medication uptakec | ||||
| 2017 | 60 183 | 307 442 623.15 | 49 365 366.28 | 258 077 256.86 |
| 2018 | 60 210 | 307 582 847.46 | 49 387 881.78 | 258 194 965.68 |
| 2019 | 60 264 | 307 855 976.85 | 49 431 737.55 | 258 424 239.30 |
| 2020 | 60 338 | 308 235 043.52 | 49 492 603.43 | 258 742 440.09 |
| 2021 | 60 417 | 308 639 780.59 | 49 557 591.14 | 259 082 189.44 |
| Secondary prevention 6.01%band progressive medication uptakec | ||||
| 2017 | 22 624 | 115 574 367.70 | 18 557 514.69 | 97 016 853.01 |
| 2018 | 56 613 | 289 208 368.22 | 46 437 533.23 | 242 770 834.99 |
| 2019 | 158 746 | 810 953 037.16 | 130 212 894.06 | 680 740 143.10 |
| 2020 | 170 255 | 869 746 737.20 | 139 653 265.43 | 730 093 471.77 |
| 2021 | 181 862 | 929 042 135.19 | 149 174 193.31 | 779 867 941.88 |
| Secondary prevention 6.01%band 100% medication uptakec | ||||
| 2017 | 181 157 | 925 438 550.12 | 148 595 573.81 | 776 842 976.31 |
| 2018 | 181 239 | 925 860 641.84 | 148 663 348.12 | 777 197 293.72 |
| 2019 | 181 400 | 926 682 793.50 | 148 795 359.15 | 777 887 434.35 |
| 2020 | 181 624 | 927 823 828.89 | 148 978 572.62 | 778 845 256.27 |
| 2021 | 181 862 | 929 042 135.19 | 149 174 193.31 | 779 867 941.88 |
| Secondary prevention 0.9%band progressive medication uptakec | ||||
| 2017 | 3388 | 17 307 309.64 | 2 778 995.54 | 14 528 314.10 |
| 2018 | 8478 | 43 309 073.44 | 6 954 039.92 | 36 355 033.53 |
| 2019 | 23 772 | 121 440 554.65 | 19 499 435.05 | 101 941 119.60 |
| 2020 | 25 496 | 130 244 935.69 | 20 913 134.59 | 109 331 801.10 |
| 2021 | 27 234 | 139 124 446.20 | 22 338 897.50 | 116 785 548.70 |
| Secondary prevention 0.9%band 100% medication uptake | ||||
| 2017 | 27 128 | 138 584 807.84 | 22 252 248.99 | 116 332 558.85 |
| 2018 | 27 141 | 138 648 016.25 | 22 262 398.22 | 116 385 618.03 |
| 2019 | 27 165 | 138 771 133.80 | 22 282 166.93 | 116 488 966.87 |
| 2020 | 27 198 | 138 942 004.33 | 22 309 603.22 | 116 632 401.11 |
| 2021 | 27 234 | 139 124 446.20 | 22 338 897.50 | 116 785 548.70 |
The model assumes that 14% of patients have poor lipid control despite optimized statin therapy; this figure includes an estimated 8% of patients with statin intolerance. The mean per-patient cost is €5511.30 for evolocumab + statin therapy and €820.26 for ezetimibe + statins.
Evolucumab therapy is associated with a lower frequency of events; however, according the results of the present study, its use is inefficient in the Spanish National Health System. The cost-effectiveness models presented here reveal an ICER of €650 000 for each cardiovascular event averted with evolocumab compared with the standard therapy. Given the lack of a cost threshold for evaluating this type of result, it is difficult to reach a firm conclusion about the cost-effectiveness of evolocumab therapy.
LimitationsA limitation of the present study is the varying level of rigor in the Diagnostic Related Groups patient classification system for calculating complications.12 The data reported here may not reflect the situation in Spain; however, any discrepancy is likely to be small. The analysis did not consider mid- and long-term costs due to complications. The survival curve modeling allowed us to project costs over a 10-year time horizon; however, it is important to recognize that the recorded data cover a follow-up of just 26 months and that there is therefore high uncertainty in the model. Because of this, there were insufficient data to model all the events considered, and an estimate of cost per quality-adjusted life-year gained would have required overly risky assumptions. We felt it important to respect this limitation rather than present cost-effectiveness data purporting to support decision making. Finally, given the lack of endpoint studies for the familial hypercholesterolemia patients, primary prevention costs and budget impact for these patients were calculated from the efficacy data for dyslipidemia patients in secondary prevention.
The FOURIER study includes a population with only moderately high mean LDL-C values at baseline (92mg/mL).3 However, the analysis showed no correlation between cardiovascular protection and the severity of baseline cholesterolemia; there was no significant difference between the protective effect for patients with LDL-C < 80mg/dL (HR = 0.80; 95%CI, 0.69-0.93) and those with LDL-C > 109mg/dL (HR = 0.89; 95%CI, 0.77-1.02).
Several long-term cost-utility analyses have evaluated the ability of PCSK9 inhibitors to improve the lipid profiles of US patients in long-term models. Although these studies are highly heterogeneous, they all report high ICER values, between 268 637 and 506 000 dollars per quality-adjusted life-year gained.27–29 In contrast, a Spanish study by Villa et al. reported ICER values between €30 893 and €42 266 per quality-adjusted life-year gained.9 The difference between these reported values may be due to the price of evolocumab, which was $14 000 to $14 600 per patient per year in the US-based studies,27–29 whereas the cost used in the study by Villa et al.9 was $4969.60 per patient per year. Moreover, the estimated cardiovascular mortality reductions in that study were based on extrapolations from LDL-C values and were much larger than the reductions reported in the FOURIER study; had the analysis been based on the FOURIER trial data, the efficiency of evolocumab would also have been lower.
Another 2 recent reports evaluated the cost-effectiveness of evolocumab from the US health system perspective, using clinical data from the FOURIER study; these studies generated ICER values of 337 729 and 450 000 dollars per quality-adjusted life-year gained.30,31
The reduction in morbidity and mortality was not as expected, for complex reasons that as yet remain unclear .28 Cardiovascular events have a multitude of causes, and pharmacological agents also have multiple effects. Reductions in cholesterol, blood pressure, and blood glucose show a clear epidemiological association with reductions in major adverse cardiovascular events; however, this association does not necessarily hold for all mechanisms of action or clinical situations,32 as for example demonstrated for metformin monotherapy vs its administration in combination with sulfonylureas in type 2 diabetes patients.33 It is therefore essential that results be confirmed in clinical endpoint studies.34 Clinical situations involving significant injury and high risk can occur in the context of only moderately elevated LDL-C, as seen among patients in the FOURIER trial; these patients may therefore be less responsive to lipid-lowering therapies than those with lower disease severity but less pronounced dyslipidemia. It should be noted that the percentage reduction in LDL-C achieved by the addition of a drug to a pre-existing therapy will be less than that achieved with the same drug given as monotherapy at baseline.35 For some drugs and clinical situations, it is possible that consensus goals for LDL-C lowering based on expert assessment of epidemiological data will not produce the hoped for clinical outcomes.
These results highlight the advisability of major price reductions for PCSK9 inhibitors.27,28,30,31,36 This would improve efficiency and reduce the budget impact. The maintenance of the current high prices for these treatments places great importance on patient selection.36 Spanish public funding criteria for PCSK9 inhitors (released before the availability of morbidity and mortality data) include LDL-C > 100mg/dL.37,38 The Spanish Society of Atherosclerosis and other organizations such as the UK NICE (National Institute for Health and Care Excellence) propose a progressive scale of LDL-C values for PCSK9 inhibitor initiation, with the threshold depending on the attributable risk in specific patient subgroups.39–42 It would be useful to have a more detailed analysis of economic and organizational factors related to PCSK9 inhibitor use, including a budget impact analysis to identify the most efficient strategy.
In any event, it is also higly recommendable to achieve the best possible lipid control before introducing PCSK9 inhibitor therapy.43 Advisable steps include reviewing treatment adherence, maximizing statin therapy effectiveness through careful drug and dose selection, improving strategies to increase tolerance, and using other available treatments such as ezetimibe. All patients placed on PCSK9 inhibitor therapy should be monitored closely, and strategies should be investigated to refine the treatment regimen as required.44
An examination of the subgroup analysis in the FOURIER study suggests that the number of averted cardiovascular events may be lower in Europe (HR = 0.91; 95%CI, 0.83-1.00) than in North America (HR = 0.77; 95%CI, 0.66-0.90).3 More detailed knowledge would be useful about factors affecting possible effect differences related to comorbidities and other risk factors, such as diabetes. Publication of the results of the ODYSSEY trial with alirocumab will provide additional information about the cardiovascular benefits of PCSK9 inhibitors.4
CONCLUSIONSThe present study increases the information available on the efficiency of PCSK9-inhibitor therapy and its usefulness and projected impact in the Spanish National Health System. The analysis presented here indicates that evolovumab therapy is currently not cost-effective in patients at high cardiovascular risk and LDL-C > 100mg/dL. In light of these findings, a major price review of PCSK9 inhibitors is warranted.
CONFLICTS OF INTERESTNone declared.
- –
Major clinical trials with PCSK9 inhibitors have shown large reductions in LDL-C. The price of these drugs was established before the availability of morbidity and mortality data, and is considerably higher than for other drugs used in cardiovascular prevention.
- –
In a clinical trial of evolocumab vs placebo in secondary prevention, a cardiovascular event was averted in 1.5% of patients (9.8% vs 11.3%) after 26 months.
- –
For the Spanish National Health System, the ICER for evolocumab at the current price was €600 000 per averted cardiovascular event at 6 months.
- –
The budget impact of introducing evolocumab for secondary prevention in Spain (2017) for patients with LDL-C > 100mg/dL would be between €32 million and €259 million.