Assessment of the cost-effectiveness of dabigatran for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation in Spain, from the perspective of the National Health System.
MethodsAdaptation of a Markov chain model that simulates the natural history of the disease over the lifetime of a cohort of 10 000 patients with non-valvular atrial fibrillation. Model comparators were warfarin in a first scenario, and a real world prescribing pattern in a second scenario, in which 60% of the patients were treated with vitamin K antagonists, 30% with acetylsalicylic acid, and 10% received no treatment. Deterministic and probabilistic sensitivity analyses were performed.
ResultsDabigatran reduced the occurrence of clinical events in both scenarios, providing gains in quantity and quality of life. The incremental cost-effectiveness ratio for dabigatran compared to warfarin was 17581 euros/quality-adjusted life year gained and 14118 euros/quality-adjusted life year gained when compared to the real world prescribing pattern. Efficiency in subgroups was demonstrated. When the social costs were incorporated into the analysis, dabigatran was found to be a dominant strategy (ie, more effective and less costly). The model proved to be robust.
ConclusionsFrom the perspective of the Spanish National Health System, dabigatran is an efficient strategy for the prevention of stroke in patients with non-valvular atrial fibrillation compared to warfarin and to the real-world prescribing pattern; incremental cost-effectiveness ratios were below the 30 000 euros/quality-adjusted life year threshold in both scenarios. Dabigatran would also be a dominant strategy from the societal perspective, providing society with a more effective therapy at a lower cost compared to the other 2 alternatives.
Keywords
Atrial fibrillation (AF), the most common cardiac arrhythmia, is the cause of a high percentage of ischemic strokes, and its importance as an etiological factor in these events increases with age.1
In Spain, the overall prevalence of AF is 4.8% in the general population,2 rising to 8.5% among patients over 60 years of age, and reaching 16.5% among those over 85 years.3
The hospital costs associated with stroke in Spain in 2004 was 1526 million euros.4 If, in addition to this cost, we considered the indirect costs and other direct non-health care costs, we would obtain estimates of the total cost of stroke similar to 5% of Spanish public health expenditures.5
The profiles of strokes in patients with AF differ from those affecting patients in sinus rhythm.6 From the clinical point of view, the former are usually more extensive and produce a greater initial neurological deficit. Moreover, the sequelae are more important. Thus, the probability of a stroke associated with AF resulting in disability is 2.23-fold higher than that of a stroke in an individual with sinus rhythm.7
This greater severity implies a longer hospital stay and a lower probability of the patient being discharged to his or her home.8
The presence of AF is also an independent risk factor for in-hospital death in patients with ischemic stroke, especially women and the elderly population,9 and this risk is greater for both in-hospital and out-of-hospital mortality.10
All these circumstances account for the greater economic and social impact of stroke with AF compared to stroke without AF.
Considering the poor outcome of this type of stroke, the management of patients with AF should include both treatment of the arrhythmia and the prevention of emboli. Evidence-based clinical guidelines11 recommend anticoagulation therapy for patients with AF who have associated embolic risk.
For the last 50 years, the only available oral anticoagulants have been vitamin K antagonists (VKA), which include warfarin and acenocoumarol. These drugs have been shown to be effective in the prevention of strokes and emboli.12, 13 However, because of their unpredictable pharmacokinetics and pharmacodynamics, and their individual and interindividual variability, added to their narrow therapeutic window, monitoring the degree of anticoagulation and periodic dose adjustment14 are required to reduce the risk of stroke or hemorrhage when the patients are not within the therapeutic range (international normalized ratio [INR]=2-3). Moreover, VKA are frequently subject to interactions with foods and drugs.
With each patient having the INR checked an average of 13 times a year, monitoring this parameter is a burden on the patients and on the health care system. Moreover, the aim is not always achieved, since it has been estimated that patients treated with VKA remain outside the therapeutic INR range nearly half the time.15
Because of the aforementioned inconveniences of VKA, many patients are unable to receive thromboprophylaxis or the treatment they receive is inadequate,16, 17, 18 meaning that their need for the prevention of stroke due to AF is not being covered.
Dabigatran etexilate is a direct thrombin inhibitor recently authorized by the European Medicines Agency19 for the prevention of stroke and systemic embolism in adult patients with non-valvular AF and with 1 or more of the following risk factors: previous stroke, transient ischemic attack or systemic embolism; left ventricular ejection fraction less than 40%; symptomatic heart failure in New York Heart Association functional class II or higher; age 75 years or older; and age 65 years or older associated with diabetes mellitus, coronary heart disease, or hypertension. Dabigatran has predictable pharmacokinetics and pharmacodynamics and a broad therapeutic window and, thus, in contrast to VKA, does not require coagulation monitoring. The prospective, randomized study, the RE-LY (Randomized Evaluation of Long-term anticoagulation therapy) trial, included 18 113 patients and compared 2 blinded doses of dabigatran etexilate (150mg or 110mg, both taken twice daily) with warfarin in terms of efficacy and safety for stroke prevention in patients with non-valvular AF.20, 21
After a median follow-up period of 2 years, the efficacy of dabigatran 150mg administered twice daily was found to be superior to that of warfarin in terms of the primary efficacy variable studied: prevention of stroke and systemic embolism (relative risk reduction [RRR], 35%). It also significantly reduced the risk of ischemic stroke (RRR, 24%), hemorrhagic stroke (RRR, 74%), vascular death (RRR, 15%), intracranial hemorrhage (RRR, 59%), life-threatening hemorrhage (RRR, 20%), and major and minor bleeding combined (RRR, 9%). The rate of gastrointestinal bleeding was higher (relative risk increase, 48%). The 110-mg dose twice daily was noninferior to warfarin with respect to the primary efficacy variable, was superior in terms of the primary safety variable, major bleeding (RRR, 20%), and significantly reduced hemorrhagic stroke (RRR, 69%), intracranial hemorrhage (RRR, 70%), life-threatening hemorrhage (RRR, 33%), major bleeding (RRR, 20%), and major and minor bleeding combined (RRR, 22%). Likewise, in terms of the net clinical benefit, the 150-mg dose was superior (RRR, 10%) to warfarin and the 110-mg dose was noninferior.
Once the efficacy and safety of an alternative strategy have been demonstrated, its efficiency in terms of costs and benefits should be evaluated to optimize the use of health care resources and patient access to the most efficient therapies.
The objective of the present study was to evaluate the cost-effectiveness of the utilization of dabigatran for the prevention of stroke and systemic embolism in adult patients with non-valvular AF with 1 or more thromboembolic risk factors, from the perspective of the Spanish health authorities.
METHODS DesignWe used a Markov model to simulate the natural history of patients with non-valvular AF over their entire lifetime. The conceptual model had been developed and reported previously,22 and has been used in other economic evaluations of dabigatran23 that have been analyzed by the agencies for health technology assessment of several countries, such as the National Institute for Health and Clinical Excellence in the United Kingdom.24
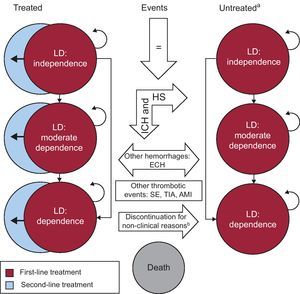
The model utilized in this study includes 23 health states (15 permanent and 8 temporary) based on the patient's level of disability, history of stroke, and treatment regimen. Figure 1 shows a diagram of the model, which takes into account 3 levels of disability: no disability (score on the Rankin scale≤225), moderate disability (Rankin 3-4), and total disability (Rankin=5). Likewise, 3 treatment regimens are considered: the initial regimen, in which the patients are treated with the therapies being analyzed; the second, involving acetylsalicylic acid; and the third, in which the patients receive no treatment.
Figure 1. Markov diagram. AMI, acute myocardial infarction; ECH, extracranial hemorrhage; HS, hemorrhagic stroke; ICH, intracranial hemorrhage; LD, level of disability; SE, systemic embolism; TIA, transient ischemic attack. aHistory of stroke was included in the model but is not shown in the diagram. bDiscontinuation due to non-major hemorrhagic events.
Once the simulation commences, each patient is susceptible to developing 1 of the clinical events considered (primary and recurrent ischemic stroke, hemorrhagic stroke, transient ischemic attack, systemic embolism, acute myocardial infarction, intracranial hemorrhage, extracranial hemorrhage) and/or can die.
In the diagram, the arrows indicate the transitions permitted between the different health states. These transitions are produced as a consequence of variations in the level of dependence of the patient or due to discontinuation of or changes in the treatment regimen, and take place in accordance with the assumptions of the model presented in Table 1. The duration of the Markov cycles was 3 months. The half-cycle correction was applied.
Table 1. Model Assumptions.
| Clinical efficacy (probabilities of experiencing events) |
| Dabigatran and warfarin: RE-LY trial post hoc analysis stratified by age |
| Acetylsalicylic acid and no treatment: meta-analysis of indirect comparisons |
| Discontinuation rates: from the original model 22 |
| Dabigatran and warfarin: according to the RE-LY trial |
| Acetylsalicylic acid: according to the BAFTA trial |
| Rates of mortality and disability, and utilities |
| Overall mortality: Spanish population by age and sex |
| Additional mortality and disability due to events: from the original model 22 |
| Utilities: from the original model 22 |
| Obtained from EQ-5D scores and incorporated according to the patient's level of dependence |
| Disutilities were associated with the development of some clinical events |
| Other premises of the model. In the base case, it is assumed that: |
| The patients receive no anticoagulation medication concomitantly with the medication included in the model |
| Dabigatran does not interact with any other medication |
| The transition of a patient to a higher level of disability is only associated with the occurrence of cerebral events (stroke or intracranial hemorrhage) |
| Patients cannot transition to lower levels of disability |
| The probability of a patient transitioning to a worse level of disability after an event is not related to a history of previous strokes |
| Patients continue to be treated with the initial medication for the rest of their lives, except in cases of authorized treatment discontinuation |
| Patients who experience an intracranial hemorrhage or hemorrhagic stroke discontinue the treatment permanently for the remainder of the simulation |
| After experiencing an intracranial hemorrhage, the patient can discontinue the treatment temporally (50% of the cases during a 3-month cycle) or permanently (the remaining 50%) |
| Temporary treatment interruptions following development of an extracranial hemorrhage are not associated with changes in the risk of the occurrence of any of the events |
| In cases of permanent treatment discontinuation for reasons other than the development of ischemic stroke or intracranial hemorrhage, 70% of the patients change to a second-line treatment regimen |
| A maximum of 2 treatment regimens is allowed per patient throughout the entire simulation |
| In the prescribing pattern scenario, the patients initially treated with acetylsalicylic acid who discontinue this treatment do not receive any other therapy as second-line treatment |
| Patients are allowed to experience only 1 of the clinical events described (with the exception of minor bleeding) or death for each 3-month cycle. Minor bleeding can occur at any time in the cycle, either alone or in association with any of the other events, although minor bleeding does not increase the risk of developing any of the other events of greater importance in that cycle |
| No association is established between death due to stroke, intracranial hemorrhage, or extracranial hemorrhage and the existence of a history of stroke |
| The efficacy of the treatments is assumed as a constant over time |
| Temporary treatment interruptions for short periods of time (1-2 weeks), which can take place following stroke or during perioperative periods, are not associated with significant impacts on the efficacy or the cost of the treatments |
| INR monitoring of the patients being treated with vitamin K antagonists (warfarin or acenocoumarol) is not associated with disutilities in addition to those derived from the level of disability of the patient or from the development of the clinical events considered |
BAFTA, Birmingham Atrial Fibrillation Treatment of the Aged; INR, international normalized ratio; RE-LY, Randomized Evaluation of Long-term anticoagulation therapy.
Source: our own data, based on Sorensen et al. 22,23
The adaptation of the model to the Spanish population was carried out by a multidisciplinary expert panel, the members of which are the authors of this manuscript. Using structured questionnaires, data were collected on the use of resources and routine clinical practice in Spain, agreed on by consensus in a face-to-face meeting and compared with those reported in the available scientific literature.
ScenariosThe first scenario considered compared the alternatives evaluated in the RE-LY trial21: dabigatran and warfarin. The second compared dabigatran with the prescribing pattern in clinical practice in Spain; in agreement with the available evidence,26 it was assumed that 60% of the population receives VKA therapy, 30% takes acetylsalicylic acid, and 10% receives no treatment at all.
In the case of dabigatran, a chain model was developed; simulating the conditions of the technical specifications, the patients began the intervention with a 150-mg dose of dabigatran 2 times a day and, upon reaching the age of 80 years, changed to a 110-mg dose twice daily, being subject in each stage to the respective probabilities of experiencing an event observed for each dose in the RE-LY trial.19
PopulationThe population was a hypothetical cohort of 10 000 patients with non-valvular AF that simulated the profile of the patients in the RE-LY trial. At the initiation of the simulation, the mean age of the patients was 69.1 years; all the patients started out with no disability and remained in the model until they made the transition to the state of “death”. The distribution of the cohort according to stroke risk and time within therapeutic INR range for the patients receiving warfarin was that of the RE-LY trial.
Mortality, Clinical Efficacy, Disability, and UtilitiesThe overall risk of mortality was that of the Spanish population according to age and sex.27
The clinical efficacy (probability of experiencing an event in the simulation), the rates of additional mortality, disability due to events and discontinuation, and utilities were taken from the original model.22
Perspective, Time Horizon, and Discount RatesThe perspective of the analysis was that of the Spanish National Health System, and the time horizon covered the lifetime of the patient (assumed maximum life expectancy, 100 years). The costs and benefits were discounted at an annual rate of 3%.
Use of Resources and CostsIn the model base case, only direct health care costs associated with the therapies evaluated were considered. All the costs are expressed in 2010 euros. The necessary cost updates were performed according to the corresponding annual Consumer Price Index.28
Medication costs were calculated on the basis of the retail price29 considering the 7.5% discount in medications covered by Spanish Royal Decree 8/2010.30
The costs of the clinical events were obtained from diagnosis-related groups31 or from the literature.32 In events with associated follow-up costs (stroke and hemorrhagic stroke), the cost associated with the level of dependence of the patient was added to the cost of the event.
The monitoring of the INR constitutes an additional cost in VKA therapy, and has been included in the cost of the medication. To estimate this, we considered the different care modalities utilized in Spain, taking into account the technique for obtaining the blood sample and the setting in which monitoring is performed, and the costs reported by de Solà-Morales et al.33 were applied. Likewise, for this calculation, we assumed that, on a general basis, anticoagulation is poorly controlled in 30% of the patients.34 Table 2 shows the calculation of the total annual cost of INR monitoring (total amount weighted according to setting). The costs included in the model are detailed in Table 3.
Table 2. Estimation of the Annual Cost of International Normalized Ratio Monitoring (2010 Euros).
| Monitoring setting and type of blood sample collection (venous or capillary) | Patients per setting, % | Annual cost according to usual patient control level | |
| Good control (70% of the patients, monitored 13 times a year) | Poor control (30% of the patients, monitored 19.5 times a year) | ||
| Hospital (10% venous) | 3 | 173.63 | 260.44 |
| Hospital (90% capillary) | 27 | 378.00 | 462.31 |
| Primary care (100% capillary) | 67 | 378.00 | 462.31 |
| Home (100% capillary) | 2 | 673.31 | 905.28 |
| Self-monitoring (100% capillary) | 1 | 883.90 | 1221.16 |
| Total | 100 | 382.83 | 472.70 |
It was assumed that 30% of the patients are usually outside the therapeutic range (poor control) and undergo 50% more international normalized ratio measurements than the average. Thus, the current cost allocations related to monitoring doubled.
Source: Spanish expert panel and de Solà-Morales Serra et al. 33
Table 3. Costs Corresponding to the Model Base Case (2010 Euros).
| Therapeutic alternative | Daily cost, retail price+VAT a | Source |
| Dabigatran 150mg (twice daily) | 3.03 | MSPSI 29 |
| Dabigatran 110mg (twice daily) | 3.03 | MSPSI 29 |
| Warfarin 2.1 mg | 0.05 | MSPSI 29 |
| Acetylsalicylic acid, weighted means of 100 mg (70%) and 300 mg (30%) | 0.10 | MSPSI 29 |
| Clinical event | Cost | Source |
| Fatal ischemic stroke | 4237.76 | MSPSI 31 |
| Ischemic stroke, independent | 4407.58 | MSPSI, 31 López-Bastida et al. b |
| Ischemic stroke, moderately dependent | 4827.18 | MSPSI, 31 López-Bastida et al. b |
| Ischemic stroke, totally dependent | 5483.06 | MSPSI, 31 López-Bastida et al. b |
| Fatal systemic embolism | 1834.94 | MSPSI 31 |
| Non-fatal systemic embolism | 1834.94 | MSPSI 31 |
| Transient ischemic attack | 2453.36 | MSPSI 31 |
| Fatal intracranial hemorrhage | 5830.96 | MSPSI 31 |
| Intracranial hemorrhage, independent | 6000.78 | MSPSI, 31 López-Bastida et al. b |
| Intracranial hemorrhage, moderately dependent | 6250.56 | MSPSI, 31 López-Bastida et al. b |
| Intracranial hemorrhage, totally dependent | 6486.84 | MSPSI, 31 López-Bastida et al. b |
| Fatal hemorrhagic stroke | 5830.96 | MSPSI 31 |
| Hemorrhagic stroke, independent | 6000.78 | MSPSI, 31 López-Bastida et al. b |
| Hemorrhagic stroke, moderately dependent | 6250.56 | MSPSI, 31 López-Bastida et al. b |
| Hemorrhagic stroke, totally dependent | 6486.84 | MSPSI, 31 López-Bastida et al. b |
| Fatal extracranial hemorrhage | 3724.68 | MSPSI 31 |
| Nonfatal, non-gastrointestinal extracranial hemorrhage | 2581.82 | MSPSI 31 |
| Nonfatal, gastrointestinal extracranial hemorrhage | 2581.82 | MSPSI 31 |
| Minor bleeding | 188.96 | Oblikue Consulting 32 |
| Fatal acute myocardial infarction | 4072.94 c | MSPSI 31 |
| Nonfatal acute myocardial infarction | 4072.94 c | MSPSI 31 |
| Associated degree of disability | Cost per every 3 months of follow-up | Source |
| Following stroke, independent | 169.82 | López-Bastida et al. b |
| Following stroke, moderately dependent | 419.60 | López-Bastida et al. b |
| Following stroke, totally dependent | 655.88 | López-Bastida et al. b |
| International normalized ratio monitoring | Annual cost | |
| Patients usually exhibiting good control | 382.83 | Table 2 |
| Patients usually exhibiting poor control | 472.70 | Table 2 |
MSPSI, Ministerio de Sanidad, Política Social e Igualdad (Spanish Ministry of Health, Social Policy and Equality); VAT, value added tax.
a With a 7.5% reduction in accordance with Spanish Royal Decree 8/2010 30 .
b Source: López-Bastida J, Oliva Moreno J, Worbes Cerezo M, Perestelo Pérez L, Serrano-Aguilar P, et al. The Social Economic Costs and Health-Related Quality of Life of Stroke Survivors in the Canary Islands, Spain. Working paper. (J. López-BAstida, personal communication, 15 November, 2011).
c It is considered that 11% are silent.
Deterministic and probabilistic sensitivity analyses were performed to confirm the robustness of the model and identify the parameters with the greatest influence on the results.
In the deterministic analysis, the parameters with the greatest uncertainty were modified; they included the time horizon, discount rate, subpopulation of patients of 80 years or older (only treated with dabigatran 100mg), and the percentage of time within the therapeutic range in INR monitoring (57.1% and 72.6%), with the same cutoff points as those utilized in a post hoc analysis of the RE-LY trial data.35
A deterministic sensitivity analysis was also carried out to observe the efficacy in 4 subgroups: patients with poor or good INR control (defined as time within the therapeutic range<57.1% or 72.6%), patients taking acetylsalicylic acid, and untreated patients, in order to examine the societal perspective. This included non-health care costs36 and assessment of the informal care received by stroke survivors with some level of dependence,37, 38 and the value added tax was deduced from the price of the medications (Table 4).
Table 4. Deemed Incremental Costs for the Deterministic Sensitivity Analysis From the Societal Perspective, According to Disability (2010 Euros).
| Independent and history of stroke | Moderately dependent | Totally dependent | Source | |
| Applied to the price of drugs | Deduction of VAT and discounts (applied to the retail price) | MSPSI 29 | ||
| Applied to the disabling event, ischemic or hemorrhagic stroke and intracranial hemorrhage | ||||
| Investment (eg, home remodeling) | 0 | 31.1 | 31.1 | Hervás-Angulo et al. 36,a |
| Public institutional assistance | 0 | 125.8 | 125.8 | Hervás-Angulo et al. 36,a |
| Applied to follow-up of stroke patients performed every 3 months | ||||
| Private institutional assistance | 0 | 76.9 | 76.9 | Hervás-Angulo et al. 36,a |
| Nursing homes and/or day care centers | 0 | 426.6 | 426.6 | Hervás-Angulo et al. 36,a |
| Informal care (hourly rate, 10.6 euros) a | 5970.1 | 9596.6 | 11 958.9 | Hidalgo et al., 37 Jiménez-Martín et al., 38 and Oliva-Moreno et al. b |
MSPSI: Ministerio de Sanidad, Política Social e Igualdad; (Spanish Ministry of Health, Social Policy and Equality); VAT, value added tax.
Estimation of the hours of informal and non-professional care, provided to stroke survivors with some degree of dependence by relatives or friends and exclusively as a consequence of a personal bond. Based on primary data from the Survey on Disabilities, Personal Autonomy, and Situations of Dependency 2008 (Encuesta sobre Discapacidades, Autonomía personal y situaciones de Dependencia 2008) (Spanish National Statistics Institute), using replacement cost methodology, censoring at 16h a day as the maximum duration of informal care and considering only the main caregiver (no additional or occasional caregivers). Based on the cited report, we calculated the average hourly rate for care in the scenarios assessed (7.67 euros and 12.71 euros), and their categories of dependence were adapted to ours as follows: non-dependence to independence with a history of stroke, moderate dependence, and the average of severe dependence and high dependence to total dependence.
a Based on the average of years 2 and 3 of the cited work.
b Oliva-Moreno J, Aranda-Reneo I, Vilaplana C, González-Domínguez A, Hidalgo-Vega A. Informal care of cerebrovascular accident survivors with activities of daily living limitations (mimeo). (J. Oliva-Moreno, personal communication, 15 November 2011).
The probabilistic analysis performed (10 000 Monte Carlo simulations) modified the values of the parameters simultaneously, according to the functions of beta distribution to describe baseline risks and utilities, log normal distribution to describe relative risks, and gamma distribution to describe costs.
ResultsIn the model employed, dabigatran reduced the number of ischemic strokes, cases of systemic embolism, transient ischemic attacks, intracranial hemorrhages, and hemorrhagic strokes compared both to warfarin (scenario 1) and to the prescribing pattern (scenario 2), but it increased the number of extracranial hemorrhages (attributable to nonfatal gastrointestinal hemorrhage) and acute myocardial infarctions (Table 5).
Table 5. Clinical Events Observed in the Overall Cohort of 10 000 Patients According to Alternative.
| Clinical events | Dabigatran | Warfarin | PP | Δ dabigatran vs warfarin | Δ dabigatran vs PP |
| Ischemic stroke | 4244 | 4407 | 5170 | –162 | –926 |
| Fatal | 1606 | 1596 | 1870 | 9 | –264 |
| Independent | 1435 | 1598 | 1841 | –164 | –406 |
| Moderately dependent | 734 | 688 | 807 | 46 | –72 |
| Totally dependent | 470 | 524 | 653 | –54 | –183 |
| Systemic embolism | 508 | 563 | 680 | –56 | –173 |
| Fatal | 2 | 2 | 2 | 0 | –1 |
| Non-fatal | 506 | 561 | 678 | –55 | –172 |
| Transient ischemic attack | 1301 | 1535 | 1674 | –234 | –372 |
| Intracranial hemorrhage and hemorrhagic stroke | 480 | 1078 | 909 | –598 | –429 |
| Fatal | 200 | 512 | 377 | –312 | –177 |
| Independent | 48 | 93 | 93 | –44 | –44 |
| Moderately dependent | 55 | 106 | 105 | –51 | –50 |
| Totally dependent | 176 | 367 | 334 | –191 | –158 |
| Extracranial hemorrhage | 4336 | 3884 | 3790 | 452 | 546 |
| Fatal | 52 | 47 | 46 | 5 | 7 |
| Non fatal, non-gastrointestinal | 3360 | 3147 | 3073 | 213 | 287 |
| Non-fatal, gastrointestinal | 924 | 690 | 672 | 234 | 251 |
| Acute myocardial infarction | 1419 | 1161 | 1267 | 257 | 152 |
| Fatal | 16 | 13 | 14 | 3 | 2 |
| Non-fatal | 1403 | 1148 | 1253 | 255 | 150 |
| Total dependence (Rankin=5) | 646 | 891 | 987 | –245 | –341 |
| Total fatal events | 1875 | 2170 | 2309 | –295 | –434 |
| Total events | 12 288 | 12 628 | 13 490 | –340 | –1203 |
PP, prescribing pattern.
In the 2 scenarios, dabigatran reduced the number of fatal events by 295 and 434, respectively, and the number of events that result in some type of disability by 250 and 463, respectively; of these disabling events avoided, 98% and 73% led to complete dependence (Rankin=5) in scenarios 1 and 2, respectively.
With regard to life years gained and quality-adjusted life years (QALY) gained, dabigatran produced a lifetime gain of 2514 life years and 2759 QALY in the first scenario and of 3625 life years and 4085 QALY in the second.
In the cost-effectiveness analysis carried out, we obtained a value of 17 581 euros/QALY gained for the comparison of dabigatran versus warfarin, and a value of 14 118 euros/QALY gained versus the prescribing pattern (Table 6).
Table 6. Results of the Cost-effectiveness Analysis in Euros per Quality-adjusted Life Year Gained per Patient.
| Per patient, for the entire time horizon | Cost of medication and monitoring* | Cost of events* | Cost of follow-up* | Total cost * | LYG | QALY | Incremental cost | Incremental QALY | ICER, (€/QALY) |
| Scenario 1 (dabigatran vs warfarin, RE-LY) | |||||||||
| Warfarin | 3475 | 3678 | 3190 | 10 343 | 11.13 | 8.45 | 4851 | 0.28 | 17 581 |
| Dabigatran | 8857 | 3409 | 2927 | 15 193 | 11.39 | 8.73 | |||
| Scenario 2 (dabigatran vs prescribing pattern) | |||||||||
| Prescribing pattern | 2178 | 3889 | 3358 | 9426 | 11.02 | 8.32 | 5769 | 0.341 | 14 118 |
| Dabigatran | 8857 | 3409 | 2927 | 15 193 | 11.39 | 8.73 | |||
ICER, incremental cost-effectiveness ratio; LYG, life years gained; QALY, quality-adjusted life years; RE-LY, Randomized Evaluation of Long-term anticoagulation therapy.
* In 2010 euros.
From the perspective of the Spanish National Health System, the changes in the incremental cost-effectiveness ratio observed in the univariate deterministic analysis in the first scenario ranged between 14 651 and 57 719 euros/QALY. In scenario 2, the cost-effectiveness ranged between 11 519 euros/QALY and 52 160 euros/QALY.
In the analysis from the societal perspective, dabigatran proved to be a dominant strategy as it showed a higher effectiveness and lower cost when compared with warfarin and with the prescribing pattern, with a reduction of 6 957 025 euros and 41 237 148 euros, respectively (Table 7).
Table 7. Results of the Deterministic Sensitivity Analysis.
| Parameter | BC value | SA value | Dabigatran vs warfarin, RE-LY | Dabigatran vs PP | ||
| ICER (€/QALY) | Change with respect to BC | ICER (€/QALY) | Change with respect to BC | |||
| BC results | 17 581 | 14 118 | ||||
| Discount rate | 3% | 0 | 15 127 | –14% | 11 971 | –15% |
| 5% | 19 348 | 10% | 15 684 | +11% | ||
| Time horizon | Patient lifetime | 5 years | 57 719 | 228% | 52 160 | +269% |
| 10 years | 32 001 | 82% | 27 829 | +97% | ||
| Relative risk of ischemic stroke with dabigatran vs warfarin | <80 years: 0.77; >80 years: 0.82 | <80 years: 0.58; >80 years: 0.51 | 13 217 | –25% | 11 519 | –18% |
| <80 years: 1.03; >80 years: 1.33 | 32 175 | +83% | 20 520 | +45% | ||
| Patients with total disability due to ischemic stroke | RE-LY (150 mg: 4.1%; 110 mg: 0.1%) | 150 mg: 13.3%, 110 mg: 14.6% | 21 475 | +22% | 16 137 | +14% |
| %TTR for INR * | 64.5% | 72.6% | 21 095 | 20% | 15 072 | +7% |
| 57.1% | 13 952 | –21% | 12 776 | –10% | ||
| Patient age at initiation, mean | 69.1 | +80 (82.9) | 24 034 | 37% | 17 501 | +24% |
| Cost of INR monitoring ( Table 3 ) | €382.8 | +30% | 14 014 | –20% | 12 672 | –10% |
| €472.7 | –30% | 21 149 | +20% | 15 564 | +10% | |
| Total health care costs | Drugs, events, and follow-up of the BC ( Table 3 ) | +20% | 21 097 | 20% | 16 666 | +18% |
| –20% | 14 651 | –17% | 11 765 | –17% | ||
| Dabigatran vs patients with ASA | 30% | 100% | Not applicable | 13 317 | –5% | |
| Dabigatran vs untreated patients | 10% | 100% | Not applicable | 7104 | –50% | |
| Societal perspective, incorporates social costs | Perspective of the Spanish National Health System | Costs as detailed in Table 6 | Dominant | Dominant | ||
%TTR, percentage time in therapeutic range; ASA, acetylsalicylic acid; BC, base case; ICER; incremental cost-effectiveness ratio; INR, international normalized ratio; PP, prescribing pattern; QALY, quality-adjusted life year; RE-LY, Randomized Evaluation of Long-term anticoagulation therapy; SA, sensitivity analysis.
* The percentages of time above and below therapeutic range of INR maintained the original ratio of the base case (INR<2=15.2% when TTR=72.6 and INR<2=23.9% when TTR=57.1).
The key parameters having the greatest effect on the incremental cost-effectiveness ratio were the time horizon, the perspective from which the analysis was carried out, the degree of INR control achieved in patients treated with warfarin, the reduction of stroke risk, and the decrease in long-term disability that dabigatran achieved with respect to both comparators, as well as the cost associated with INR monitoring in the patients being treated with warfarin (Table 3).
In the probabilistic sensitivity analysis, considering a willingness-to-pay threshold of 30 000 euros/QALY gained,39 dabigatran would be a cost-effective strategy vs warfarin in 96.4% of the simulations, and in 99.9% of the simulations vs the prescribing pattern (Figure 2). In the comparisons by subgroups, dabigatran proved to be a cost-effective strategy in 99.4% and 99.3% of the simulations in the case of patients with poor and good INR control, respectively, and in 99.5% and 99.9% in the case of patients taking acetylsalicylic acid and those receiving no treatment, respectively.
Figure 2. Acceptability curves according to the scenario of the comparison. A: dabigatran vs warfarin in the Randomized Evaluation of Long-term anticoagulation therapy trial. B: dabigatran vs the prescribing pattern. RE-LY, Randomized Evaluation of Long-term anticoagulation therapy.
DiscussionIn this chain model, dabigatran reduced the number of events compared with both warfarin and the prescribing pattern in routine clinical practice, resulting in gains in patients’ quantity and quality of life.
The substantial reduction of the number of cases of hemorrhagic stroke and of fatal intracranial hemorrhage was the major factor contributing to the gain in life years observed in the dabigatran arm. Likewise, in this simulation, dabigatran achieved an overall reduction in the ischemic strokes, intracranial hemorrhages, and hemorrhagic strokes that cause disability (Rankin≥3) of 15% compared to warfarin and 24% compared to the prescribing pattern in Spain, circumstances that are associated with a decrease in the costs of long-term treatment and care.
In all the comparisons in the deterministic analysis, the values of the incremental cost-effectiveness ratio were lower than the reference threshold of 30 000 euros/QALY gained,39 except for the 5-year and 10-year time horizons in scenario 1 and the 5-year time horizon in scenario 2. The latter finding is explained by the chronicity of AF and the benefits received over the patients’ lifetime. Because of the consequences of strokes with respect to medium- and long-term disability, the benefits of the treatments considered in the context of the patient's lifetime horizon need to be evaluated, and this is the ideal analysis.
With a cost-effectiveness threshold of 30 000 euros/QALY gained, the probability of dabigatran being an efficient strategy is 96.4% compared to warfarin, and 99.9% compared to the prescribing pattern, a finding that supports the robustness of the results obtained.
Limitations and StrengthsImportantly, there are a number of limitations and possible biases in this economic evaluation. For example, the data from clinical trials have limitations that can determine the external validity of the model. However, the scenario in which dabigatran is compared to routine prescription in Spain may constitute a closer approximation to real-world clinical practice.
We have identified limitations inherent in the adaptation of the model to the Spanish context. First, the disparate sources of the information on health care costs obliged us to carry out a careful selection of the values, grouping the information according to levels of disability and opting for more conservative alternatives. Second, the data on utilities employed in the model refer to the United Kingdom due to the limited references on utilities in the Spanish population and the impossibility of adapting them to the levels of disability following stroke that the design demanded. Despite this circumstance, the probabilistic sensitivity analysis was carried out using different values for the costs and utilities, and there was no evidence that these variations affected the robustness of the results.
Finally, although the anticoagulation therapy employed in Spain is acenocoumarol, rather than warfarin, we consider the 2 drugs to be perfectly interchangeable in terms of both efficacy and safety, and of the resource use they involve.
One strength of this economic evaluation is the follow-up of the disability caused by ischemic stroke or intracranial hemorrhage, an issue of vital importance given the elevated cost of long-term care, which is not usually evaluated in other economic studies.40
A second strength of the present report concerns the conservative assumptions adopted with respect to INR control. For both scenarios, a time within therapeutic range of 64.4% was assumed, a value observed in the RE-LY trial, when, in real-world clinical practice, it may be lower. Equally, the model produced no decreases in the utility of INR monitoring.
Notably, the model was designed before results from the RE-LY trial had been obtained, and the specifications of the economic analysis were established in parallel to the clinical development. Likewise, its development enabled us to include the results of the RE-LY trial in the simulation, patient by patient, rather than the group as a whole,40 and to derive results on stroke according to the levels of disability.
Seven economic evaluations of dabigatran have been published to date.23, 40, 41, 42, 43, 44, 45, 46 In the adaptations of this same model to the Canadian,23 United Kingdom42, and Danish46 contexts, dabigatran proved to be a cost-effective strategy vs both warfarin and the local prescribing pattern. In the United States, Kamel et al.,45 taking a societal perspective and a cost of dabigatran therapy per day of 6.75 dollars concluded that it is a cost-effective alternative for patients with previous stroke or transient ischemic attack.
The other 3 publications40, 41, 43, 44 concluded that dabigatran 150mg administered twice daily was cost-effective only in certain subgroups analyzed. However, these 3 reports incorporated a few hypotheses that favor warfarin over dabigatran and increase the observed incremental cost-effectiveness ratio: they factored in a cost of dabigatran higher than the list price (treatment cost of 8 dollars/day or higher),40, 43, 44 considered the risk of ischemic stroke and of intracranial hemorrhage as being independent of age,43 or employed lower rates of disability and costs of intracranial hemorrhage, when dabigatran markedly reduces the risk of intracranial hemorrhage.41 Pink et al.41 assigned to intracranial hemorrhage, an event that can produce a greater disability than stroke, a rate of permanent disability of only 0.0524, vs 0.233 for stroke, and assigned a low cost to intracranial hemorrhage, similar to that of extracranial hemorrhage. In contrast, Freeman et al.40, 44 and Shah et al.43 evaluated the 150-mg and 110-mg doses of dabigatran separately. The evaluation of the 110-mg dose twice daily is not applicable in the context of the United States, as it has not been authorized by the U.S. Food and Drug Administration. We consider that, since the results of the evaluation presented here were obtained with the chain model, they better meet the technical specifications of the drug and real-world prescribing patterns.
For their part, different agencies for health technology assessment (United Kingdom, Scotland, Denmark, Sweden, Australia, and Canada) have evaluated dabigatran in terms of this indication and have considered it to be cost-effective for the entire population.
VKA constitute a therapeutic option that has been on the market for 50 years; these drugs are inexpensive, but are less effective and/or safe and have serious limitations compared to dabigatran. First, the reduction of the risk of ischemic and hemorrhagic stroke achieved with dabigatran offers greater quantity and quality of life and, moreover, this drug does not require INR monitoring. Together, these aspects result in a reduction of the associated costs. Thus, this evaluation cannot be reduced to the standpoint of hermetic budgets based merely on a comparison of the pharmaceutical cost.
The analysis from a societal perspective included direct non-health care costs (nursing homes, adaptations to home care, caregivers, etc.) that, not being financed by the Spanish National Health System, could be partly supported by Spanish Social Security funds. Due to methodological difficulties and a lack of sufficient evidence, certain costs, such as those incurred by the patient as a result of INR monitoring, the opportunity cost in terms of the productive time of survivors with some degree of dependence, and the value of the life lost, were not included.
According to a recent report, the economic impact on hours of informal care given to stroke survivors in Spain would range between 6183.57 and 10 246.83 million euros (corresponding to 2008).37, 38 Incorporating these data and other direct non-health care costs35 into our analysis, we observed that dabigatran is associated with greater effectiveness and lower costs compared to the other 2 alternatives, and therefore, a saving for society. Thus, dabigatran is a dominant strategy in both scenarios.47
Despite the limitations mentioned above, the premises adopted in the present model appear to be reasonable and conservative, and the results of the sensitivity analyses confirm the robustness of the model and the results obtained.
The conclusive results with regard to efficacy and safety demonstrated in the RE-LY trial and its subsequent subanalyses support the view that dabigatran is indicated as a first-line treatment in oral anticoagulation therapy for the prevention of stroke in patients with non-valvular AF.11, 48
The results of the present economic evaluation show that, considering a willingness-to-pay threshold of 30000 euros/additional QALY,39 dabigatran etexilate is a cost-effective therapy for patients with non-valvular AF in Spain.
ConclusionsFrom the perspective of the Spanish National Health System, dabigatran is an effective strategy for stroke prevention in patients with non-valvular AF when compared to warfarin and to the prescribing pattern in routine clinical practice. From the societal perspective, dabigatran would also be a dominant strategy, as it would offer society greater effectiveness at lower costs than the other 2 alternatives.
Conflicts of interestAll the authors have made a substantial contribution to the design and development of the study, according to the international recommendations in this regard. Two of the authors (NGR and VB) are employees of Boehringer Ingelheim. Another author (IO) is a member of PORIB (Pharmacoeconomics & Outcomes Research Iberia), a consultancy organization specialized in economic evaluation that has provided consultancy services to Boehringer Ingelheim in connection with this study. However, this circumstance has in no case influenced the results reported here.
Acknowledgments
The authors thank Anuraag Kansal and Sonja Sorensen of United BioSource Corporation, Maryland, United States.
Received 16 February 2012
Accepted 6 June 2012
Corresponding author: Prat de la Riba s/n, Sector Turó Can Matas, 08173 Sant Cugat del Vallès, Barcelona, Spain. virginia.becerra@boehringer-ingelheim.com