The world is currently in the midst of the pandemic due to coronavirus disease 2019 (COVID-19), and Spain has reported the world's highest mortality per million inhabitants. COVID-19 is generally known to cause severe respiratory syndrome; however, reports are now emerging of a wider variety of other relevant manifestations. Some of the most concerning are cardiovascular,1 including acute myocardial injury, myocarditis, arrhythmia, and numerous cases of thromboembolic pulmonary disease. Only a few case reports of thrombotic arterial complications have been published, despite the hypercoagulable state associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),2,3 possibly due to the novelty and limited understanding of this health emergency.
Acute coronary syndrome (ACS) can be triggered by respiratory infections. Kwong et al.4 reported a 6-fold increase in the incidence of ACS in the week after influenza diagnosis compared with a control period defined as 1 year before and 1 year after the infection. A short-term increase in the risk of ACS could be presumed present and attributable to COVID-19 infection.
We describe the case of a 59-year-old physician working in an outpatient emergency department and collecting nasopharyngeal swabs for polymerase chain reaction (PCR) testing in patients with clinical suspicion of COVID-19. The physician was admitted for ACS with ST-segment elevation in the lower leads during the previous 3.5hours. The patient had hypertension, poorly controlled type 2 diabetes (glycated hemoglobin 12.2%), and no history of substance abuse. On arrival, ECG showed inferolateral ST-segment elevation with a mirror image of ST depression in the right precordial leads. On admission, vital signs were blood pressure, 150/100mmHg; heart rate, 82 bpm; weight, 107kg; height, 183cm, and body mass index, 31.94. Baseline oxygen saturation was 92%, and the lowest estimated ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) was 257, a result consistent with mild acute respiratory distress syndrome.
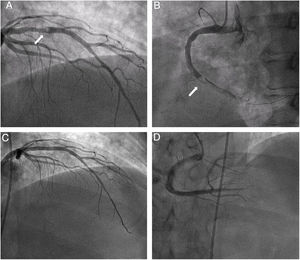
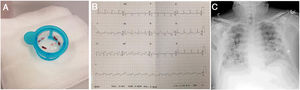
Primary angioplasty revealed a severe lesion with abundant thrombotic content in the right coronary artery (RCA) and occlusion of the posterior descending (PD) and left anterior descending (LAD) arteries, with a moderate proximal lesion showing a filling defect consistent with thrombus. Thrombotic material was aspirated from the RCA, and a direct drug-eluting stent was implanted, with the distal PD artery remaining occluded. The proximal LAD was subsequently revascularized with another direct stent (figure 1A-D, figure 2A, and ). Tirofiban was administered due to the high thrombotic burden and embolization in the distal PD. Door-to-balloon time was approximately 60minutes. The next day, ECG showed persistent ST-segment elevation of 1.5mm in the lower leads and in V4 to V6, as well as Q wave revealing inferior necrosis and first-degree atrioventricular block (figure 2B).
Following primary angioplasty and a more detailed medical history, the patient described symptoms consistent with COVID-19 (dry cough, low-grade fever, headache, and asthenia) lasting at least 3 days. Chest X-ray showed bilateral coalescent alveolar opacities, predominantly subpleural, with an interstitial pattern of increased density in the perihilar regions (figure 2C). Laboratory tests showed lymphocytopenia (0.73×103/μL), as well as elevated C-reactive protein (135.6mg/L), D-dimer (1513 ng/mL), ferritin (1239 ng/mL), and interleukin 6 (41.3 pg/mL). The disseminated intravascular coagulation (DIC) score according to the International Society on Thrombosis and Haemostasis (ISTH) scale was 3 (inconclusive for DIC). In view of the current COVID-19 pandemic, specific PCR testing for SARS-CoV-2 was performed and was positive.
The patient received conventional treatment for ACS (antithrombotic therapy with aspirin, prasugrel, and enoxaparin at anticoagulant doses during hospitalization and for an additional week due to the high thrombotic burden and suspicion of hypercoagulable state), high-flow oxygen therapy, hydroxychloroquine, and antibiotics (ceftriaxone/azithromycin).
Respiratory progress was satisfactory, and the patient was discharged after 10 days (admission on 1 April and discharge on 10 April 2020). On 17 April, follow-up PCR for SARS-CoV-2 was still positive. The patient is currently asymptomatic.
Unfortunately, the presence of active atherosclerotic plaques could not be characterized by intracoronary imaging studies using optical coherence tomography or intravascular ultrasound, due to limitations on material resources during the current COVID-19 outbreak intended to prevent contagion to medical staff and patients. However, atherosclerotic plaque rupture or erosion was the most likely cause of our patient's event, in view of the presence of obstructive coronary lesions, cardiovascular risk factors (particularly diabetes mellitus with very poor metabolic control), absence of any emboligenic foci (telemetry-recorded sinus rhythm throughout the 10-day hospital stay; transthoracic echocardiogram showing inferior akinesia with normal left ventricular ejection fraction, absence of intraventricular thrombus, morphologically normal valves, absence of proximal ascending aorta abnormalities), absence of thrombocytosis (platelets on admission, 387×103/μL), and absence of substance abuse.
Furthermore, no associated factors were found to suggest hereditary thrombophilia, as the patient was older than 50 years, had no personal or family history of thromboembolism, and had no history of recurrent thrombosis at unusual sites, such as splenic veins or the central nervous system.5 However, a complete thrombophilia study was not performed due to the risk of false positives during the acute phase, particularly in the context of active inflammation and infection, and the study was postponed for 2 months.
This clinical case indicates that the inflammatory/prothrombotic state associated with SARS-CoV-2 infection is related not only to thromboembolic disease or the microvascular territory, but may also entail a higher risk of atherosclerotic plaque rupture in the arterial system, as previously reported with other respiratory viruses, but not with SARS-CoV-2 until now.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.05.021