Heart disease associated with cancer and cancer therapies is a growing concern for clinical cardiologists.1 The European Guidelines for atrial fibrillation (AF) do not recommend differential treatment for cancer patients, and the same anticoagulation criteria are applied as for the general population.2 The risk of embolism and bleeding can be altered by the presence of cancer, and the CHA2DS2-VASc and HAS-BLED risk scales have not been validated specifically in cancer patients.
There is limited evidence supporting use of direct-acting oral anticoagulants (DOACs) in cancer patients with AF. Pivotal trials of DOACs excluded patients with short life expectancy and those with thrombocytopenia (platelets <100 000/μL). Therefore, no specific data have been generated on the safety and efficacy of DOACs in patients with AF and cancer.
Breast cancer is highly prevalent and is often treated with chemotherapy, which has been associated with a higher incidence of AF. The prolonged survival of patients with breast cancer enables extended follow-up.
Our primary objective was to assess whether there are differences in time to onset of ischemic and bleeding events in patients with breast cancer and nonvalvular AF according to risk profile and antithrombotic strategy. Other objectives were to describe our sample and calculate the risk scores to assess their validity for predicting events.
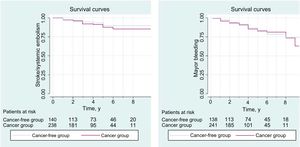
This study was a retrospective, observational, multicenter study in 9 tertiary hospitals in Spain. Patients diagnosed with nonvalvular AF and breast cancer between January 2011 and January 2018 were included from oncology and cardiology clinics (regardless of cancer stage). Valvular AF and/or mechanical prostheses were exclusion criteria. The sample was compared with a cohort of women with AF but without cancer who consecutively attended cardiology clinics. In total, 465 women were included: 312 with AF and breast cancer (cancer cohort) and 153 with AF but no cancer (cancer-free cohort). The 2 groups were compared with the chi-square test or the Student t test for categorical or continuous variables with a parametric distribution, respectively. Survival was analyzed using Cox regression to determine the predictive power of the CHA2DS2-VASc and HAS-BLED scales in this population. Kaplan-Meier curves were used to compare time to ischemic or bleeding events in patients with cancer and cancer-free patients (Figure 1). Hazard ratios (HR) were analyzed with corresponding 95% confidence intervals (95% CI). Statistical analysis was performed with the SPSS program, version 22.
Clinical data were collected and the risk scores were calculated for both groups. Embolic events included stroke and thromboembolism. Bleeding events included intracranial or gastrointestinal bleeding, epistaxis, and the development of anemia.
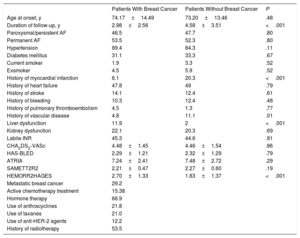
The baseline characteristics of the patients are shown in the Table 1. Overall, 97.4% of the patients had an indication for anticoagulation,2 although 15.5% of the cancer group and 11.3% of the control group were not receiving therapy (P=.005). The use of DOACs was lower in the cancer group (16% vs 25.3%, P=.004). During follow-up, 26.2% of the patients underwent a switch in antithrombotic treatment (from vitamin K antagonist to DOAC, 15.1%).
Baseline Characteristics of the Sample
| Patients With Breast Cancer | Patients Without Breast Cancer | P | |
|---|---|---|---|
| Age at onset, y | 74.17±14.49 | 73.20±13.46 | .48 |
| Duration of follow-up, y | 2.98±2.56 | 4.58±3.51 | <.001 |
| Paroxysmal/persistent AF | 46.5 | 47.7 | .80 |
| Permanent AF | 53.5 | 52.3 | .80 |
| Hypertension | 89.4 | 84.3 | .11 |
| Diabetes mellitus | 31.1 | 33.3 | .67 |
| Current smoker | 1.9 | 3.3 | .52 |
| Exsmoker | 4.5 | 5.9 | .52 |
| History of myocardial infarction | 6.1 | 20.3 | <.001 |
| History of heart failure | 47.8 | 49 | .79 |
| History of stroke | 14.1 | 12.4 | .61 |
| History of bleeding | 10.3 | 12.4 | .48 |
| History of pulmonary thromboembolism | 4.5 | 1.3 | .77 |
| History of vascular disease | 4.8 | 11.1 | .01 |
| Liver dysfunction | 11.9 | 2 | <.001 |
| Kidney dysfunction | 22.1 | 20.3 | .69 |
| Labile INR | 45.3 | 44.6 | .91 |
| CHA2DS2-VASc | 4.48±1.45 | 4.46±1.54 | .86 |
| HAS-BLED | 2.29±1.21 | 2.32±1.29 | .79 |
| ATRIA | 7.24±2.41 | 7.48±2.72 | .29 |
| SAMETT2R2 | 2.21±0.47 | 2.27±0.60 | .19 |
| HEMORR2HAGES | 2.70±1.33 | 1.83±1.37 | <.001 |
| Metastatic breast cancer | 29.2 | ||
| Active chemotherapy treatment | 15.38 | ||
| Hormone therapy | 66.9 | ||
| Use of anthracyclines | 21.8 | ||
| Use of taxanes | 21.0 | ||
| Use of anti-HER-2 agents | 12.2 | ||
| History of radiotherapy | 53.5 |
AF, atrial fibrillation.
Values expressed as percentage or mean±SD.
The presence of breast cancer was not associated with an increase in embolic or bleeding events. In the cancer group, 11% experienced an embolic event compared with 13.2% in the cancer-free group (log-rank test, 0.71; P=.72). In the cancer group, 15.9% experienced a bleeding event compared with 18.2% in the cancer-free group (log-rank test, 0.73; P=.74).
No differences were observed between groups in the predictive power of the risk scores. The CHA2DS2-VASc score was useful for predicting bleeding events (HR, 1.4; 95% CI, 1.2-1.6; P <.001). The discriminatory power, measured by the area under curve, was greater in the cancer group (0.69) than in the control group (0.53), with c-statistic values of 0.67 and 0.56, respectively. The HAS-BLED score was equally useful for predicting bleeding events in the 2 groups (HR, 1.5; 95% CI, 1.3-1.8; P <.001). The area under curve in the cancer group (0.67) and the cancer-free group (0.64) were similar whereas the c-statistic was greater in the cancer-free group (0.60 vs 0.75, respectively). These results are in line with similar studies.3
In the analysis by subgroups of the ROCKET study4 and RELY study,5 bleeding risk was 2 to 6 times higher in cancer patients. Breast cancer does not confer a particular tendency for bleeding, and in these studies, the few patients with cancer included those with other cancers such as colorectal cancer, which has a higher risk of bleeding.
Our study has several limitations, as it has an observational design, with data extracted from hospital records. Patients with more advanced cancer are less represented. The group of patients with AF without cancer was selected from cardiology clinics (and so may be subject to selection bias).
In conclusion, the presence of breast cancer was not associated with a higher incidence of embolic or bleeding events in patients with AF. A history of breast cancer did, however, point to worse antithrombotic treatment for patients with AF, that is, lower use of DOAC and a higher percentage of patients without anticoagulation despite having an indication for therapy. The CHA2DS2-VASc and HAS-BLED risk scores predict embolic and bleeding events (respectively) in patients with breast cancer and AF, with no differences in predictive power with the general population. These patients should follow the clinical guidelines for the general population in terms of anticoagulation.
FUNDINGUnrestricted grant from Abbott.