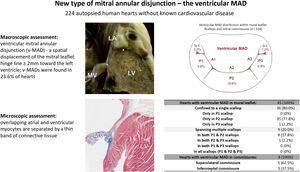
The aim of this study was to investigate a new variation of the atrial wall-mitral annulus-ventricular wall junction along the mural mitral leaflet and commissures: the ventricular mitral annular disjunction (v-MAD). This new variant is characterized by spatial displacement of the mitral leaflet hinge line by more than 2mm toward the left ventricle.
MethodsWe examined a cohort of autopsied human hearts (n=224, 21.9% females, 47.9±17.6 years) from patients without known cardiovascular disease to identify the presence of v-MAD.
ResultsMore than half (57.1%) of the hearts showed no signs of MAD in the mural mitral leaflet or mitral commissures. However, v-MAD was found in 23.6% of cases, located within 20.1% of mural leaflets, 2.2% in superolateral commissures, and 1.3% in inferoseptal commissures. V-MAD was not uniformly distributed along the mitral annulus circumference, with the most frequent site being the P2 scallop (19.6% of hearts). The v-MAD height was significantly greater in mural leaflets than in commissures (4.4 mm±1.2 mm vs 2.1 mm±0.1 mm; P<.001). No specific variations in mitral valve morphology or anthropometrical features of donors were associated with the presence or distribution of v-MADs. Microscopic examinations revealed the overlap of the thin layer of atrial myocardium over ventricular myocardium in areas of v-MAD.
ConclusionsOur study is the first to present a detailed definition and morphometric description of v-MAD. Further studies should focus on the clinical significance of v-MAD to elucidate whether it represents a benign anatomical variant or a significant clinical anomaly.
Keywords
Mitral annular disjunction (MAD) is a variant of the atrial wall-mitral annulus-ventricular wall junction alignment characterized by a spatial displacement of the mitral leaflet hinge line directed toward the left atrial (LA) wall.1 The annular displacement shifted toward the atrial wall (atrial mitral annular disjunction [a-MAD] may be present in the mural mitral leaflet [all scallops]) and both commissures.2,3 A recent study by our group revealed that a-MADs are noticeable in 20% of healthy hearts; they appeared in 12.1% of mural leaflets, 9.8% of all superolateral commisures, and 5.8% of all inferoseptal commissures.1 Mitral disjunction may be recognized as a normal structural variant and a predisposing factor for adverse cardiac events.1,4,5 While cardiologists have long ignored MAD, it currently arouses widespread interest due to its clinical implications. The presence of a-MAD was linked with mitral valve leaflet prolapse and with an increased risk of ventricular arrhythmias.6
The foundational descriptions of a-MAD and the diverse configurations of the mitral annulus were first introduced by Henle in his seminal work “Handbuch der systematischen Anatomie des Menschen” in 1876.7 In 1986, Hutchins et al. conducted a histological study of the mitral annulus region in patients with mitral valve prolapse and described the range of variation in the anatomy of the mitral annulus, identifying different relationships between the LA wall, mitral leaflet attachment line, and the left ventricular (LV) wall (atrial wall-mitral annulus-ventricular wall junction). These authors focused on a-MAD as abnormal displacement of the mural mitral leaflet attachment line onto the LA wall, away from the LV myocardium, and indicated a potential relationship between a-MAD and floppy mitral valve using 900 autopsied hearts.8 Their finding was rebutted by Angelini et al.,9 who concluded that MAD was a normal anatomical variation of the left atrioventricular junction. What is even more intriguing and has gone unnoticed until now is that Hutchins et al. also documented additional variations in the mitral annulus structure. They described the atrium-valve junction attached well below the atrial aspect of the ventricle but did not formally name, measure, or elaborate on this structural variant.8 The same ventricular-shifted disjunction was also illustrated by Henle, but also without any particular focus on this variant.7 In a previous study, we analyzed mitral annular displacement toward the left atrium.1
Intrigued by these historical observations and the apparent oversight of such variations, we decided to closely investigate the variants of the mitral annulus hinge line, focusing on displacements directed toward the LV: the ventricular mitral annular disjunction (v-MAD), a so far undefined and unexplored variant of the mitral valve complex.
METHODSThis study was conducted at the Department of Anatomy, Jagiellonian University Medical College, Krakow, Poland. Ethics approval was granted by the Bioethical Committee of the Jagiellonian University, Krakow, Poland (approval no. 1072.6120.169.2022), ensuring compliance with the ethical standards of the 1975 Declaration of Helsinki.
Study populationA total of 224 autopsied human hearts (21.9% from female donors) with a mean age of 47.9±17.6 years were analyzed. Samples were obtained during routine forensic medical autopsies performed at the Department of Forensic Medicine, Jagiellonian University Medical College, Krakow, Poland. Demographic data, including sex, age, body weight, and height, were collected. Exclusion criteria were rigorously applied to omit cases with a history of heart surgeries or grafts, evident cardiac or major vascular anomalies, heart trauma, macroscopic signs of cadaver decomposition, valvular diseases (including mitral valve prolapse), arrhythmias, or instances of suspected sudden cardiac death.
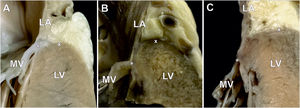
Macroscopic assessmentInitially, hearts were carefully excised from the chest cavity, weighed, and then preserved in a 10% formalin solution for up to 2 months, pending detailed examination. The LA was routinely opened (between pulmonary vein ostia) to expose the mitral valve. Mitral leaflet variations were investigated based on our previous study.10 Measurements included the aorto-mural diameter and intercommissural diameter of the mitral valve annulus. The mitral valve annulus region was then examined along the mural mitral valve leaflet and both commissures. To achieve this, the area was longitudinally sectioned at the center of each scallop (including any accessory scallops) and at the level of each mural leaflet indentation, and at both commissure levels. Altogether, each heart was assessed for v-MAD at a minimum of 9 locations through longitudinal sections. The macroscopic assessment then focused on investigating the mutual relationships between atrial myocardium, ventricular myocardium, and the mitral valve annulus in the search for v-MAD. In the classic arrangement (where MAD is not present [no-MAD]), the insertion point of the mitral annulus should be situated at the border between the atrial and ventricular walls, with no significant displacement of the mitral leaflets’ hinge line (both in the mural leaflet and commissures) toward the LA or LV (figure 1A).1 We defined the v-MAD as a spatial displacement of the mitral leaflet hinge line toward the LV wall, with a disjunction of ≥ 2mm (figure 1B,C).
Photographs of autopsy heart specimens showing longitudinal sections through the atrial wall-mitral annulus-ventricular wall junction. A: mitral valve with the classic type of mitral valve hinge line (no mitral annular disjunction). B and C: ventricular mitral annular disjunction type with visible spatial displacement of the mitral leaflet hinge line toward the LV. The mitral leaflet insertion point (asterisk) is located precisely between the LA and LV myocardium. The cross (x) indicates the highest point of the LV myocardium. LA, left atrium; LV, left ventricle; MV, mitral valve.
If v-MAD was detected, its location within the mural mitral valve leaflet and commissures was precisely described. Additionally, the height of the v-MAD was measured as the maximal distance between the ventricular wall-mitral leaflet junction and the top of the LV myocardium (along the endocardial surface). Macroscopic measurements were performed using 0.03mm precision electronic calipers YT-7201 (YATO, Poland). To ensure accuracy and mitigate observer bias, 2 independent investigators performed the measurements with the final value being the average of the 2 readings.
Microscopic assessmentFollowing the initial macroscopic evaluation, a detailed histological analysis was conducted. Representative samples from each identified type (no-MAD [n=12] and v-MAD [n=12]) underwent routine histological processing. These samples were embedded in paraffin, and 4-μm thick sections were prepared and affixed to super adhesive glass slides (Super Frost Plus White Adhesion slides, Epredia, United States) for staining. To elucidate the tissue architecture, sections were stained with hematoxylin and eosin, complemented by Masson Trichrome Staining Kit (Sigma-Aldrich, United States) to highlight collagen fibers and muscle cells. The microscopic structure of the atrioventricular junction was captured by scanning the entire stained slides with SLIDEVIEW VS200 (Olympus Life Science, United States) and retrieving the images using OlyVIA v.3.4.1 software (Olympus Life Science, United States).
Statistical analysisData were analyzed using IBM SPSS Statistics. Categorical variables are presented as numbers (No.) or percentages, and quantitative variables as mean±standard deviation. Normal distribution was assessed with the Shapiro-Wilk test. Differences between normally distributed quantitative parameters were evaluated with the Student t test, while nonnormally distributed quantitative data were investigated using the Mann-Whitney U test. Differences between categorical variables were determined using the chi-square test of independence or Fisher's exact test if the number of observations in one category was below 5. For multiple comparisons, the nonparametric Kruskal-Wallis test with post-hoc Dunn test and Bonferroni correction was applied to compare values between groups. Correlation coefficients were calculated to assess statistical dependence between measured parameters. A P-value<.05 was deemed to be statistically significant.
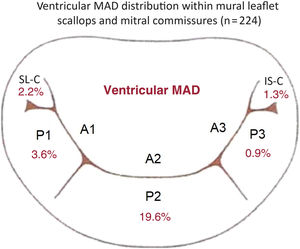
RESULTSThe classic mitral leaflet hinge line pattern (no-MAD) was observed in 57.1% of hearts, while v-MAD was identified in 23.6% of them. In the remaining hearts, a-MAD (displacement toward the atrial wall) was present. Notably, a single instance featured both v-MAD and a-MAD within the same heart. v-MAD was present in 20.1% of all mural leaflets examined, 2.2% of superolateral commissures, and 1.3% of inferoseptal commissures (figure 2). No instances of concurrent mural leaflet and commissural v-MADs were noted. The presence of v-MAD was not uniformly distributed along the mitral annulus circumference, with disjunctions never appearing along the entire mural mitral leaflet (table 1). As much as 80.0% of mural leaflet v-MADs were located within 1 scallop, and the rest (20.0%) were distributed across 2 adjacent scallops (table 1). The most common site for ventricular disjunctions was the P2 scallop (19.6% of the hearts exhibited v-MAD in the P2), followed by the P1 scallop (3.6% of hearts with v-MAD in P1) and the P3 scallop (0.9% hearts with v-MAD in P3). v-MAD was not identified in any accessory scallops of the mural leaflet (accessory mural scallops [1 or 2] were present in 19.0% of all studied hearts).
A diagram representing the distribution of v-MAD across the mural mitral valve leaflet scallops (P1, P2, and P3) and mitral commissures based on data from the entire study cohort (n=224). IS-C, inferoseptal commissure; SL-C, superolateral commissure; v-MAD, ventricular mitral annular disjunction.
Distribution of ventricular mitral annular disjunction within the mural mitral leaflet and mitral commissures
| Number of hearts with v-MAD in mural leaflet | 45 (100) |
| Confined to a single scallop | 36 (80.0) |
| Only in P1 scallop | 0 (0) |
| Only in P2 scallop | 35 (77.8) |
| Only in P3 scallop | 1 (2.2) |
| Spanning multiple scallops | 9 (20.0) |
| In both P1 and P2 scallops | 8 (17.8) |
| In both P2 and P3 scallops | 1 (2.2) |
| In both P1 and P3 scallops | 0 (0) |
| In all scallops (P1, P2, and P3) | 0 (0) |
| Number of scallops with v-MAD | 54 (100) |
| In P1 scallop | 8 (14.8) |
| In P2 scallop | 44 (81.5) |
| In P3 scallop | 2 (3.7) |
| Number of hearts with v-MAD in commissures | 8 (100) |
| Superolateral commissure | 5 (62.5) |
| Inferoseptal commissure | 3 (37.5) |
v-MAD, ventricular mitral annular disjunction.
The data are presented as No. (%).
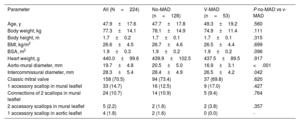
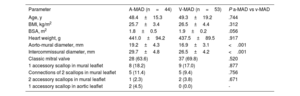
The mean height of the v-MAD was measured at 4.4 mm±1.2mm, with no significant height variations among different scallops (P1, 4.3 mm±0.9mm; P2, 4.5 mm±1.1mm; P3, 3.1 mm±1.1mm; P=.06). The mean height of v-MADs in commissures was 2.1±0.1mm and was significantly smaller than that in v-MADs found in the mural leaflet (P<.001). No significant differences or correlations were found regarding the donor's sex, age, weight, height, body mass index, body surface area, or heart weight in relation to the occurrence of v-MAD or its height (all P>.05). The aorto-mural and intercommissural diameter of the mitral valve were significantly smaller in hearts with v-MAD than in the no-MAD group, with a P-value <.05 (table 2). Additionally, no specific variations in mitral valve leaflet morphology, such as variations in scallops of the mural mitral leaflet, were associated with the presence or distribution of v-MAD (table 2). When comparing anthropometric and morphometric data between a-MAD and v-MAD groups, we noticed that both the aorto-mural and intercommissural diameters of mitral valve were smaller in the hearts with v-MAD than in donors with a-MAD, with a P-value <.001 (table 3).
Characteristic of donors and comparisons of the mitral valves with ventricular mitral annular disjunction and with classic mitral valve hinge line pattern
| Parameter | All (N=224) | No-MAD (n=128) | V-MAD (n=53) | P no-MAD vs v-MAD |
|---|---|---|---|---|
| Age, y | 47.9±17.6 | 47.7±17.8 | 49.3±19.2 | .560 |
| Body weight, kg | 77.3±14.1 | 78.1±14.9 | 74.9±11.4 | .111 |
| Body height, m | 1.7±0.2 | 1.7±0.1 | 1.7±0.1 | .315 |
| BMI, kg/m2 | 26.6±4.5 | 26.7±4.6 | 26.5±4.4 | .699 |
| BSA, m2 | 1.9±0.3 | 1.9±0.2 | 1.9±0.2 | .096 |
| Heart weight, g | 440.0±99.6 | 439.9±102.5 | 437.5±89.5 | .917 |
| Aorto-mural diameter, mm | 19.7±4.8 | 20.5±5.0 | 16.9±3.1 | <.001 |
| Intercommissural diameter, mm | 28.3±5.4 | 28.4±4.9 | 26.5±4.2 | .042 |
| Classic mitral valve | 158 (70.5) | 94 (73.4) | 37 (69.8) | .620 |
| 1 accessory scallop in mural leaflet | 33 (14.7) | 16 (12.5) | 9 (17.0) | .427 |
| Connections of 2 scallops in mural leaflet | 24 (10.7) | 14 (10.9) | 5 (9.4) | .764 |
| 2 accessory scallops in mural leaflet | 5 (2.2) | 2 (1.6) | 2 (3.8) | .357 |
| 1 accessory scallop in aortic leaflet | 4 (1.8) | 2 (1.6) | 0 (0.0) | - |
BMI, body mass index; BSA, body surface area; v-MAD, ventricular mitral annular disjunction; no-MAD, mitral annular disjunction not present.
The data are presented as No. (%) or mean±standard deviation.
Donor characteristics and comparisons of the mitral valves with ventricular mitral annular disjunction and with atrial mitral annular disjunction
| Parameter | A-MAD (n=44) | V-MAD (n=53) | P a-MAD vs v-MAD |
|---|---|---|---|
| Age, y | 48.4±15.3 | 49.3±19.2 | .744 |
| BMI, kg/m2 | 25.7±3.4 | 26.5±4.4 | .312 |
| BSA, m2 | 1.8±0.5 | 1.9±0.2 | .056 |
| Heart weight, g | 441.0±94.2 | 437.5±89.5 | .917 |
| Aorto-mural diameter, mm | 19.2±4.3 | 16.9±3.1 | <.001 |
| Intercommissural diameter, mm | 29.7±4.8 | 26.5±4.2 | <.001 |
| Classic mitral valve | 28 (63.6) | 37 (69.8) | .520 |
| 1 accessory scallop in mural leaflet | 8 (18.2) | 9 (17.0) | .877 |
| Connections of 2 scallops in mural leaflet | 5 (11.4) | 5 (9.4) | .756 |
| 2 accessory scallops in mural leaflet | 1 (2.3) | 2 (3.8) | .671 |
| 1 accessory scallop in aortic leaflet | 2 (4.5) | 0 (0.0) | - |
A-MAD, atrial mitral annular disjunction; BMI, body mass index; BSA, body surface area; v-MAD, ventricular mitral annular disjunction.
The data are presented as No. (%) or mean±standard deviation.
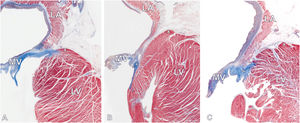
Histological sections revealed the microscopical arrangement of the v-MAD (figure 3). In the no-MAD type (classic configuration), the atrial and ventricular myocardium are adjacent to each other, divided by a discrete band of connective tissue (epicardial adipose tissue), and the mitral valve annulus formed from a rich fibrocollagenous layer of tissue sits exactly at the atrioventricular junction (figure 3A). In contrast, the microscopic image of the v-MAD shows the overlap of the thin layer of the atrial myocardium over the ventricular myocardium, creating a macroscopically visible image of the displacement (figure 3B, C). The overlapping atrial and ventricular myocytes are separated by a thin band of connective tissue. The mitral valve annulus, situated on the ventricular myocardium, is more compact than that observed in the classic arrangement and a-MAD type.
Histological images (Masson's trichrome staining) showing longitudinal sections through the mitral atrioventricular junction with 2 distinct types of junction arrangements. A: mitral valve with no-MAD (classic type). The mitral leaflet insertion point (asterisk) is located between the LA and LV myocardium. B and C: mitral valve with ventricular mitral annular disjunction. Significant spatial displacement of the mitral leaflet hinge line (asterisk) toward the LV with overlapping atrial and ventricular walls can be observed. The cross (x) indicates the highest point of the LV myocardium. LA, left atrium; LV, left ventricle; MV, mitral valve.
The left atrioventricular junction is one of the most complicated mechanical structures in the human body and is referred to as the mitral valve complex, which has numerous structural variations.10,11 Contrary to the traditional view of the mitral annulus as a complete, circular ring of dense connective tissue anchoring the 2 leaflets, this structure is more complex and often discontinuous. The mural part of the mitral annulus covers the inferolateral aspect of the left atrioventricular orifice and extends from the superolateral to the inferoseptal commissure. The remaining circumference of the “annulus” is completed by the aorto-mitral continuity, which supports the aortic mitral leaflet.12,13 Anatomically, the mitral annular complex consists of 4 components: the atrial wall, the leaflet hinge line, the crest of the free wall of the LV, and the epicardial adipose tissue. A string of connective tissue around the mural part of the mitral orifice is intended to bind these components together but is often discontinuous.13 Such incomplete “annuli” vary in thickness and density along the mural leaflet and commissures.13,14 In parts where the fibrous cord is absent, the mural leaflet is hinged directly to the atrial wall, to the ventricular wall, or at the junction of atrial and ventricular myocardium.15
Our study is the first to define and describe in detail a new type of atrial wall-mitral annulus-ventricular wall junction arrangement that may be found along the mural mitral leaflet and mitral commissures, which we named v-MAD. The new variant, characterized by a spatial displacement of the mitral leaflet hinge line toward the LV was present in 23.6% of the hearts examined (figure 4). Our previous study in the same population focused on the characteristics of a-MAD (annular displacement toward the LA), whose prevalence was estimated at 19.6%.1 Collectively, these findings offer a comprehensive view of the structural variations of the mitral valve annulus hinge line, where the classic type of the alignment (no-MAD) is present in 57.1% of hearts. v-MAD is the most common variant of the disjunction (present in 23.6% of cases) followed by a-MAD (present in 19.6% of hearts), with extremely rare cases of a-MAD and v-MAD coexistence in the same heart (0.45%). Notably, the height of v-MAD was found to be greater than that of a-MAD (P<.001).1 Interestingly, hearts with v-MAD had significantly smaller dimensions of the mitral annulus than no-MAD and a-MAD cases. This may be partly explained either by the “deeper placement” of the annulus in the LV chamber (ventricular displacement) or greater thickness of the left ventricular wall basal segments in hearts with v-MAD, which may narrow the dimensions of the mitral orifice. Nevertheless, this finding requires further research and observation.
Central illustration. A new type of mitral annular disjunction, termed ventricular MAD. The cross (x) indicates the highest point of the left ventricle myocardium, and the asterisk the mitral leaflet insertion point. IS-C, inferoseptal commissure; LA, left atrium; LV, left ventricle; MV, mitral valve; SL-C, superolateral commissure; v-MAD, ventricular mitral annular disjunction.
V-MAD has not been thoroughly explored in anatomical studies, and its clinical implications have not been investigated. Given the scarcity of autopsied or imaging studies on this topic, comparing our results with other morphological observations is challenging. Henle, later followed by Hutching, originally introduced the concept of “the atrium-valve junction attached well below the atrial aspect of the ventricle”.7,8 In the following decades, such a configuration was rarely noticed, although it was occasionally described in scientific works.13 However, no significant anatomical or clinical studies have examined this type of mitral valve attachment. Therefore, the clinical significance of the v-MAD remains a mystery.
In cases of v-MAD, the mural leaflet is inserted below the ventricular crest. Macroscopically, it appears as if it is directly inserted into the ventricular myocardium. However, microscopical analysis shows that in v-MAD, there is a significant overlap of the atrial myocardium over the ventricular myocardium rather than a true disjunction between them. Both myocardial layers are separated only by a thin band of connective tissue (figure 3). Therefore, v-MAD can resemble the inward pulling of the LA wall into the LV. In such a configuration, the epicardial connective tissue deeply penetrates the space between the crest of the ventricular myocardium and LA wall, reaching up to the hinge line of the mural leaflet. This type of mitral annulus displacement may be referred to by some as a “micro-Ebstein's malformation” of the mitral valve leaflet.16–21 However, to prevent confusion in the terminology and clinical interpretation of our findings, we have chosen to refer to this arrangement as a second variant of mitral annulus disjunction (next to the a-MAD variant). There are 2 primary arguments for this: a) Ebstein's malformation refers to the incorrect location of the right atrioventricular valve, not the mitral valve; 22and b) in v-MAD, as in a-MAD, there is a true displacement of the classic location of the mitral hinge line but shifted toward the ventricle, not the atrium. Nevertheless, both micro-Ebstein's malformation and MAD may be associated with arrhythmia.
A-MAD is recognized as an anatomical variation that may pose a risk of adverse clinical outcomes.1 The high prevalence of a-MADs and v-MADs in structurally normal hearts suggests that it is a variant of the anatomical norm. However, associations between a-MAD and mitral valve prolapse, ventricular arrhythmias, and sudden cardiac death have been confirmed.4–6,23–28 It should be emphasized that MAD is interspersed around the circumference of the junction supporting the mural leaflet of the mitral valve and commissures.1–3,29,30 Even within the same heart, we can observe varying positions of the leaflet hinge line in relation to the walls of the LA and LV, and MADs are typically sectional, with disjunctions that usually occupy a small section of the valve perimeter. Interestingly, however, cases in which both a-MAD and v-MAD coexist are extremely rare (< 0.5%). Future studies should strongly concentrate on defining thresholds for clinically meaningful MADs.
LimitationsThis study has several limitations. Our morphological and morphometric evaluations were conducted on formaldehyde-fixed autopsied material, potentially impacting the obtained measurements; however, it is worth noting that previous studies have demonstrated that paraformaldehyde fixation does not significantly affect the dimensions of human heart tissue.31,32 Additionally, we cannot assess the dynamic behavior of the studied area during the cardiac cycle due to the nature of the study design and the autopsied tissue used. Future functional anatomical studies are needed to visualize the behavior of the mitral leaflet hinge line throughout the heart cycle. Furthermore, our study population was comprised solely of Caucasian individuals, limiting the applicability of the findings across different ethnic groups. Finally, our analysis was focused on healthy hearts, especially those without significant mitral valve diseases, which may restrict the applicability of our results to patients with mitral diseases or other cardiac abnormalities. Despite these limitations, our study offers a detailed morphometric analysis and depiction of v-MAD, contributing to the understanding of this complex anatomical feature.
CONCLUSIONSOur study introduces a new type of atrial wall-mitral annulus-ventricular wall junction along the mural mitral leaflet and commissures: vMAD. This variant was present in 23.6% of the healthy hearts examined and predominantly found in the mitral mural leaflet but was also noticeable in both mitral commissures. V-MADs are generally sectional disjunctions, which do not usually extend beyond one of the scallops or commissures. The most common site for v-MADs is the P2 scallop. Mural leaflet v-MADs are significantly larger than those placed within the commissures. Future research should investigate the clinical implications of v-MAD to elucidate whether v-MAD is a benign anatomical variant or a clinically significant anomaly.
- -
MAD is a variant of the atrial wall-mitral annulus-ventricular wall junction alignment. The annular displacement shifted toward the atrial wall, known as a-MAD, may be present in the mural mitral leaflet and both commissures.
- -
Mitral disjunction may be recognized as a normal structural variant and a predisposing factor for adverse cardiac events.
- -
This study introduces a new type of the atrial wall-mitral annulus-ventricular wall junction along the mural mitral leaflet and commissures, termed v-MAD, characterized by a spatial displacement of the mitral leaflet hinge line by more than 2mm toward the LV.
- -
We examined a large sample of autopsied human hearts (n=224) and found v-MAD in 20.1% of all mural leaflets examined, 2.2% of superolateral commissures, and 1.3% of inferoseptal commissures. This study offers detailed morphological and histological characteristics of v-MAD.
None.
ETHICAL CONSIDERATIONSEthics approval was granted by the Bioethical Committee of the Jagiellonian University (Approval no. 1072.6120.169.2022), ensuring compliance with the ethical standards of the 1975 Declaration of Helsinki. Written consent was not required, as this is an autopsy study. Our Bioethics Committee waived the need for consent from donors. We collected hearts during routine forensic medical autopsies performed at the Department of Forensic Medicine (Jagiellonian University Medical College, Krakow, Poland) only from deceased persons who did not express objection when alive and only if the family did not express objection. Possible sex/gender biases have been taken into account in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tool was used to prepare this work.
AUTHORS’ CONTRIBUTIONSA. Krawczyk-Ożóg oversaw the concept, design, data acquisition, data analysis and interpretation, and drafted the manuscript. M.K. Hołda acquired the data, images, and critically revised and approved the manuscript. J. Batko and A. Dziewierz analyzed and interpreted the data, and critically revised the manuscript. K. Jaśkiewicz and B. Zdzierak analyzed and interpreted the data. W. Zasada acquired the data. K. Gil oversaw histological processing and analysis. J. Hołda oversaw data acquisition, critical revision, approval, and supervision of the manuscript. All authors approved the manuscript.
CONFLICTS OF INTERESTThe authors have no conflicts of interest to disclose related to the present manuscript.