Atrial fibrillation (AF) is common in patients with end-stage chronic kidney disease (ESCKD), with a prevalence of 7% to 24%.1 AF leads to a 6-fold increase in the risk of stroke in hemodialysis patients1 and a 5-fold increase in the hazard of bleeding.2 Several reports have found that warfarin is associated with an increased risk of bleeding and no prevention or even a higher risk of stroke in this population.3 Few publications have evaluated the effects of direct oral anticoagulants (DOACs) in patients with ESCKD4 and there have been no studies of their clinical benefit in this scenario. The recently reported (and prematurely stopped) Renal-AF trial (NCT02942407) found no significant differences between apixaban and warfarin in terms of stroke and bleeding rates.
In this scenario, percutaneous left atrial appendage occlusion (LAAO) appears to be an appealing alternative therapy.5 The LAAO with Watchman Device in Patients with Non-valvular Atrial Fibrillation and End-stage Chronic Kidney Disease on Hemodialysis (EPIC06-WATCH-HD) study (NCT03446794) is a prospective, multicenter, observational study to investigate the reduction on stroke and bleeding events after LAAO with Watchman 2.5 or Watchman FLX (Boston Scientific, USA) during a clinical follow up of 24 months.
We adopted an adaptive approach to determine the sample size for this study. The incidence of stroke and major bleeding events in this population were approximately 5% and 9% per year, respectively.1,2 Using simulation, we initially hypothesized that 95 patients would be necessary to demonstrate a 50% reduction in these rates with a power of 80% (α=0.05), considering a 15% of potential missing participants per year. An interim analysis was planned when 50 patients were recruited.
Inclusion and exclusion criteria are summarized in table 1. The primary efficacy objective is a composite endpoint including embolic (transient ischemic attack, stroke, systemic embolism) and major bleeding (Bleeding Academic Research Consortium> 2) events at 2 years. The secondary safety endpoints are: periprocedural major adverse events (mortality, stroke, systemic embolism, pericardial tamponade, pericardial effusion requiring intervention), and device-related adverse events at 2 years (thrombosis, significant residual leak> 5mm, embolization).
Eligibility criteria
| Inclusion criteria |
| Age> 18 y. |
| End-stage chronic kidney disease (glomerular filtration rate <15 mL/min) on hemodialysis. |
| History of AF (paroxysmal, persistent, permanent). |
| CHA2DS2-VASc score ≥ 2 or active oral anticoagulation due to AF. |
| HAS-BLED score ≥ 3 or a history of major bleeding (Bleeding Academic Research Consortium> 2). |
| Patient provision of written informed consent. |
| Exclusion criteria |
| Life expectancy <2 y. |
| Indication for oral anticoagulation other than from AF. |
| Severe pericardial effusion. |
| Previous percutaneous closure of atrial septal defect. |
| Intracardiac thrombus. |
| Severe hepatic dysfunction with spontaneous INR> 1.5. |
AF, atrial fibrillation; INR, international normalized ratio.
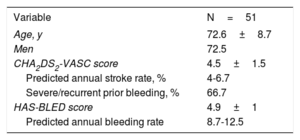
The study was approved by local ethics committees and all patients signed an informed consent form before inclusion. Enrollment in this multicenter registry started in 2017 and 51 patients have already been included at 12 sites in Spain. Baseline characteristics are detailed in table 2. LAAO was successfully performed in all patients except 1 (detection of thrombus into the LAA) with 2 transient complications: 1 case of anaphylactic shock (potentially related to contrast) and 1 LAA perforation successfully treated with LAAO (no pericardial drainage was needed). All the patients were discharged free from the safety endpoint; only 8 patients remained on oral anticoagulation at discharge.
Baseline characteristics
| Variable | N=51 |
|---|---|
| Age, y | 72.6±8.7 |
| Men | 72.5 |
| CHA2DS2-VASC score | 4.5±1.5 |
| Predicted annual stroke rate, % | 4-6.7 |
| Severe/recurrent prior bleeding, % | 66.7 |
| HAS-BLED score | 4.9±1 |
| Predicted annual bleeding rate | 8.7-12.5 |
The results are expressed as percentage or mean±standard deviation.
During follow-up, device-related thrombosis was found in 2 patients by transesophageal echocardiography. Both patients were treated with enoxaparin, with subsequent disappearance of thrombus. Only 1 patient showed a significant peridevice leak> 5mm. During a median follow up of 246 [interquartile range, 169-375] days, the following events were registered: 1 patient (1.96%) had an ischemic non-embolic lacunar stroke (while on aspirin treatment); 3 patients (5.88%) had a major bleeding event, 1 case related to arteriovenous fistula and 2 cases of gastrointestinal bleeding (all the events while on dual antiplatelet therapy); and 10 patients (19.6%) died (only 1 death was attributed to cardiovascular cause: intestinal ischemia, without evidence of embolism).
Given the observed event rates, the sample size was recalculated to 136 patients to achieve an 80% power (α=0.05) for detecting a 50% reduction in combined events. The increase with respect to our prespecified sample size was mainly due to the higher-than-expected mortality ratio and bleeding rates.
LAAO seems feasible and safe in patients with ESCKD. Interestingly, to date, no embolic events have been documented in our series. Likewise, an Italian retrospective series of hemodialysis patients reported no embolic events after LAAO at 2 years.6 Previously, 2 randomized clinical trials aimed to compare LAAO and medical therapy in this population (STOP-HARM [NCT02885545] and WatchAFIB [NCT02039167]), but both were prematurely terminated due to a slow recruitment rate. The observed bleeding rate (5.8%) was slightly higher than hypothesized (4.5%), but was lower than that predicted by the HAS-BLED score (8.7%-12.5% per year).
The observed mortality rate (19.6%) exceeded the estimated 15%, although most of the causes were not related to ischemic or bleeding events. In this r egard, a recently reported meta-analysis3 reported a death rate of 43.4% to 52.5% in patients with AF on hemodialysis with a mean follow-up of 2.6±1.4 years. In the Italian series,6 the patients on hemodialysis treated with LAAO had a 2-year death rate of 22.3% (95% confidence interval, 13.4-35.9).
Although this study has the intrinsic limitations of a single-arm, observational study (no control group, different antithrombotic regimes), it could serve as hypothesis-generator.
Patients with AF on hemodialysis represent a high-risk cohort for both ischemic and bleeding events. In this scenario, LAAO seems to be a safe strategy. Accurate selection of candidates is critical to gain the maximal benefit of treatment, avoiding futility.
FUNDINGThis study was partially supported by an unrestricted grant from Boston Scientific, Ref. ISRCAR00217.
AUTHORS’ CONTRIBUTIONST. Benito-González: conceptualization, methodology, validation, formal analysis, data curation, writing—original draft, writing—review and editing, visualization. A. Quirós: methodology, formal analysis, writing—original draft, writing—review and editing. F. Torres-Saura, J.M. Ruiz-Nodar, and I. Cruz-González: investigation, writing—review and editing. A. Pérez de Prado: conceptualization, methodology, validation, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration, funding acquisition.
CONFLICTS OF INTERESTI. Cruz-González, J.M. Ruiz-Nodar, and A. Pérez de Prado are consultants and proctors for Boston Scientific. The other authors indicate no conflicts of interest.
The authors wish to acknowledge the contribution of the participating centers and principal investigators: José Antonio Fernández Díaz, Ernesto Valero, Xavier Freixa, Luis Nombela, Germán Calle, Roberto del Castillo, Dabit Arzamendi, Jose F. Díaz, Mario Prieto, and Felipe Fernández-Vázquez.