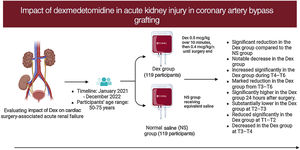
To evaluate the impact of dexmedetomidine impact on cardiac surgery-associated acute kidney injury (CSA-AKI), kidney function, and metabolic and oxidative stress in patients undergoing coronary artery bypass grafting with heart-lung machine support.
MethodsA randomized double-masked trial with 238 participants (50-75 years) undergoing coronary artery bypass grafting was conducted from January 2021 to December 2022. The participants were divided into Dex (n=119) and NS (n = 119) groups. Dex was administered at 0.5 mcg/kg over 10minutes, then 0.4 mcg/kg/h until the end of surgery; the NS group received equivalent saline. Blood and urine were sampled at various time points pre- and postsurgery. The primary outcome measure was the incidence of CSA-AKI, defined as the occurrence of AKI within 96hours after surgery.
ResultsThe incidence of CSA-AKI was significantly lower in the Dex group than in the NS group (18.26% vs 32.46%; P=.014). Substantial increases were found in estimated glomerular filtration rate value at T4–T6 (P<.05) and urine volume 24hours after surgery (P<.01). Marked decreases were found in serum creatinine level, blood glucose level at T1–T2 (P<.01), blood urea nitrogen level at T3–T6 (P<.01), free fatty acid level at T2–T3 (P<.01), and lactate level at T3–T4 (P<.01).
ConclusionsDex reduces CSA-AKI, potentially by regulating metabolic disorders and reducing oxidative stress.
Registered with the Chinese Clinical Study Registry (No. ChiCTR2100051804).
Keywords
Cardiac surgery-associated (CSA) acute kidney injury (AKI) is a significant postoperative complication in cardiac surgery patients, characterized by high morbidity, mortality, and a poor prognosis, thus posing a major health care challenge. It is an independent risk factor for chronic renal disease and increased in-hospital mortality, with a prevalence of 28% to 49% after procedures such as coronary artery bypass grafting (CABG) and heart valve replacement under extracorporeal circulation.1–3 The primary cause of CSA-AKI is renal tubular damage from ischemia-reperfusion, renal oxygen supply-demand imbalance, and inflammation linked to cardiopulmonary assistance.4,5 However, effective prevention and mitigation strategies for CSA-AKI remain under investigation.
Renal tubule epithelial cell impairment is key in ischemia-reperfusion-induced AKI, affecting renal structure and function.6,7 Renal tubule epithelial cells rely heavily on fatty acid oxidation for energy metabolism, with mitochondria playing a central role.8,9 Investigating the metabolic basis and regulation of key glycolysis enzymes during transient ischemia and hypoxia is crucial due to its effects on renal tubule epithelial cell energy deficit and potential damage.10–14 Dexmedetomidine (Dex), a highly selective α2-adrenoceptor agonist, is extensively applied in clinical anesthesia and intensive care units due to its optimal sedative, analgesic and organ-shielding properties.15–19 Given the scarcity of existing literature, the present study aimed to elucidate whether Dex mitigates ischemia-reperfusion-induced AKI by modulating fatty acid and glucose metabolism and reducing oxidative stress in patients undergoing CABG under cardiopulmonary bypass (CPB). Dex may have real benefits by exerting oxidant and anti-inflammatory effects, inhibiting antidiuretic hormone at the renal collecting ducts, and maintaining cortical blood circulation.
METHODSPatientsThis forward-looking randomized double-masked placebo-regulated investigation was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Bengbu Medical College (Approval No. 2021KY013) and registered with the Chinese Clinical Study Registry (No. ChiCTR2100051804). The study was performed in strict compliance with the Helsinki Guidelines, the principles of the Universal Declaration on the Human Rights and guidelines established by the World Health Organization Research Ethics Review Committee. Written informed consent was obtained from the participants before surgery.
Patients undergoing CABG under CPB in the Cardiac Surgery Department of the First Affiliated Hospital of Bengbu Medical Collegewere were recruited from January 2021 to December 2022. The eligibility criteria were as follows: a) minimum age ≥ 18 years; b) evaluation level II or III of The American Society for Anesthesia classification or New York Heart Association (NYHA) stage II to III; c) sufficient kidney function, defined as an estimated glomerular filtration rate (eGFR) of >60mL/min/1.73 m2; and d) provision of agreed written agreement.
The exclusion criteria were as follows: a) individuals with insufficient liver and kidney function, characterized as a eGFR of<60mL/min/1.73 m2; b) cardiogenic shock; c) a history of surgery or infection within the previous 2 weeks; d) malignant tumors; e) use of nephrotoxic drugs before the operation or during the study; f) an inability to complete the study due to poor adherence; g) cardiac catheterization within 7 days before surgery; h) contrast-induced AKI.
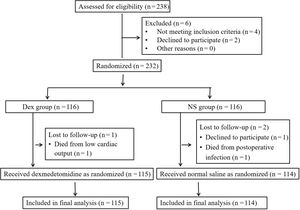
Randomization and blindingThe patients were randomly divided into either the Dex group or the normal saline (NS) group using an online randomization tool.20 The ratio was set to 1:1, with block sizes of 2 and 4 and a list length of 232. The list length was based on the sample size estimate and consequently 238 patients were recruited to account for possible dropouts. However, 6 patients were excluded, resulting in 232 patients for randomization. The randomly assigned results were placed in opaque envelopes and retained by special personnel. Dex and NS solutions were prepared by a designated research group member based on the randomization results. The Dex and NS solutions were prepared and drawn into 50mL syringes, which were marked only with random codes. At the end of all the tests, the code in the envelope was matched to the code on the syringe to reveal the grouping status. To avoid bias due to subjective factors relating to the participant and investigator, the participants, study operators and outcome evaluators were not informed of the grouping status or syringe contents (figure 1 and figure 2).
Prior to surgery, patients fasted for 8hours. Intravenous-inhaled general anesthesia was administered, with continuous monitoring of electrocardiography, non-invasive blood pressure, pulse oximetry saturation, and bispectral index. Peripheral venous access and radial artery catheterization under local anesthesia were established for continuous invasive blood pressure monitoring. Anesthesia induction involved midazolam (0.06mg/kg), etomidate (0.3mg/kg), sufentanil (2μg/kg), and cisatracurium (0.3mg/kg) administered intravenously in sequence. Tracheal intubation was performed, maintaining an inhalation oxygen concentration of 75% to 100%, oxygen flow at 1.0 to 1.5 L/min, tidal volume at 8 to 10mL/kg, ventilation rate at 10 to 12 cycles/min, and PetCO2 at 35 to 45mmHg. Right internal jugular vein cannulation facilitated central venous tension monitoring, blood sampling, and drug administration. Anesthesia maintenance included intermittent inhalation of sevoflurane, intravenous infusion of propofol and remifentanil, and intermittent intravenous injection of sufentanil and cisatracurium, with narcotic drug dosages adjusted to maintain the bispectral index value between 40 and 60. Cefazolin was used as the antibiotic prophylaxis for all patients unless they were allergic or resistant to this drug. Hydroxyethyl starch was used as the colloid of choice for all patients unless they were allergic to this drug or had intolerance.
InterventionsThe patients assigned to the Dex group were administered an initial dose of Dex (0.5μg/kg, intravenously delivered for 10minutes), followed by a constant drip at a rate of 0.4μg/kg/h until the end of surgery. The patients assigned to the NS group received NS treatment instead of Dex, with the same administration as for Dex. During the operation, blood pressure and heart rate (HR) were maintained by intravenous administration of vasoactive drugs (dopamine, ephedrine, norepinephrine, epinephrine, nitroglycerin, etc), HR-regulating drugs (atropine, isoproterenol, esmolol, etc), and dilatation. Severe bradycardia was defined as HR <40 beats per minute, and the number of patients who experienced severe bradycardia and the number of times they received HR-regulating drugs were recorded.
Baseline dataWe recorded age, sex, body mass index, American Society of Anesthesiologists and New York Heart Association grade, European cardiovascular surgery risk factor score, left ventricular ejection fraction and the complications of hypertension, hyperlipidemia and diabetes in the 2 groups. Presurgical treatments, such as angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists and nonsteroidal anti-inflammatory agents, were also documented.
Intraoperative and postoperative conditionsPostoperative: operating time, CPB time, ascending aorta occlusion time, relapse of the cardiac condition, fluid (crystal, colloidal and allogeneic red blood cell) infusion volume, urine volume, blood loss (total blood loss minus autologous blood transfusion) and dopamine and nitroglycerin dosages were recorded. Postoperative: The number of cases of CSA-AKI, AKI stage, 24-hour postoperative urine volume, postoperative ventilatory support, length of stay in the intensive care unit, and length of postoperative hospitalization were recorded.
Perioperative hemodynamic changesMean arterial pressure, central venous pressure, HR and hemoglobin values were recorded before the induction of anesthesia (T0), at the end of the operation (T1), 12hours after the operation (T2), 24hours after the operation (T3), 48hours after the operation (T4), 72hours after the operation (T5) and 96hours after the operation (T6). CPB flow and perfusion parameters, such as pump flow, mean perfusion pressure and hematocrit, were also recorded during the operation. Minimal mean arterial pressure was defined as the lowest value of mean arterial pressure during the operation.
Markers of renal function, glucose and fatty acid metabolism, and oxidative stressArterial blood, venous blood and urine specimens were gathered at intervals T0–T6. Levels of fasting plasma glucose, lactic acid and hemoglobin in arterial blood were measured using a blood gas analyzer (Radiometer, United States). Free fatty acid, serum creatinine (SCr), blood urea nitrogen, urinary kidney damage molecule-1, urinary cystatin C concentration and superoxide dismutase activity were detected using a biochemical analyzer (Beckman Coulter, United States). eGFR was calculated using Yimai Tong medical software based on an improved simplified method from 2006 by Yimai Tong medical computing software (Beijing Medlive Technology Co, China).21 The formula estimates the glomerular filtration rate using factors such as SCr concentration, age, and sex.
Levels of serum malondialdehyde were determined using the thiobarbituric acid method, and the serum total antioxidant capacity (Nanjing Jiancheng, China) was determined by colorimetry. Serum reactive oxygen species (Shanghai Jianglai, China) and urinary neutrophil gelatinase-associated lipocalcin (Nanjing Jiancheng, China) were determined by enzyme-linked immunosorbent testing. All samples were prepared, stored and tested in strict accordance with the manufacturer's operating instructions.
Currently, AKI diagnosis and staging criteria mainly include those found in the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines, the RIFLE (Risk of Renal Dysfunction, Injury to Kidney, Failure or Loss of Kidney Function), and End-stage kidney disease diagnosis and staging criteria and the Acute Kidney Injury Network staging criteria. Among them, the most commonly used are the KDIGO diagnosis and staging criteria for AKI (2012), and the diagnosis and staging criteria for CSA-AKI in this study refer to these standards.22
OutcomesThe primary outcome measure was the incidence of CSA-AKI, defined as the occurrence of AKI within 96hours after surgery. Secondary outcomes were the levels of SCr, blood urea nitrogen, free fatty acid, fasting plasma glucose and lactic acid; eGFR values and 24-hour urine volume after surgery, malondialdehyde, reactive oxygen species, total antioxidant capacity, urinary kidney damage molecule-1, urinary neutrophil gelatinase-associated lipocalcin and urinary cystatin C levels, superoxide dismutase activity and postoperative mechanical ventilation time, postoperative intensive care unit stay, number of postoperative hospitalization days, perioperative HR, and other observational indicators. eGFR was estimated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula, which is predicated on SCr, age, and sex. The formula is as follows:
eGFR=141×least (SCr / κ, 1) α×greatest (SCr / κ, 1)-1.209×0.993Age×1.018 (if woman), where SCr is SCr (mg/dL), κ is 0.7 for women and 0.9 for men, α is -0.329 for women and -0.411 for men, least signifies the least value of SCr/κ or 1, and greatest signifies the greatest value of SCr/κ or 1.
Sample sizeThe study population was estimated online by a biometrics network.23 A difference test was conducted using the comparison rate and 2-group design and was verified using PASS 15.0 (NCSS, LLC, United States) statistical software.
A retrospective cohort study reported a 28.9% incidence of AKI after CABG.24 The efficacy of Dex in the treatment of AKI after CABG is unknown. This study hypothesized that Dex would reduce the incidence of AKI from 28.9% to 14.45% (ie, a relative reduction of 50%). The parameters were set as follows: a (test level)=0.05, 1-β (test efficacy, power)=0.80, Pc (control rate value)=0.289, Pt (test rate value)=0.1445, Nt (test sample size): Nc (control sample size)=1:1. The sample size was estimated at n=226, Nt=113 and Nc=113. Considering that surgical or patient factors contributed to the loss of follow-up, this study anticipated a loss rate of 5%. Therefore, the study aimed to enrol a total of 238 participants, with 119 in each group.
Statistical analysisThe data were examined and processed using SPSS 25.0 analysis software. All evaluations were conducted in a modified per-protocol population. Data following a Gaussian distribution were denoted as x¯±s, and a separate-sample Student test was employed for comparisons between collectives. Data not following a Gaussian distribution were depicted by M (25th percentile, 75th percentile), and the Wilcoxon rank-sum nonparametric examination was used for comparisons between groups. Data on the proportion of components are reported as percentages, and the chi-square test was applied for comparisons between groups. A threshold of P<.05 was acknowledged as a statistically significant difference. Based on the results of the analyses, statistical charts were generated using GraphPad Prism 9.3.1 program.
RESULTSPatient recruitmentA total of 238 patients scheduled to receive CABG under CPB were recruited for eligibility screening based on the sample size estimate. Six patients were excluded due to insufficient renal function or chronic kidney disease before surgery (n=4) or refusal to participate (n=2). Thus, a total of 232 patients were eligible for inclusion. The Dex cohort (n=116) and the NS cohort (n=116) were randomly divided using a computerized numerical method. Of these 232 patients, 3 were excluded due to withdrawal requests (n=1) or death due to severe postoperative infection and low cardiac output (n=2). Finally, a total of 229 patients were enrolled and analyzed, including 115 patients in the Dex cohort and 114 in the NS cohort (figure 2).
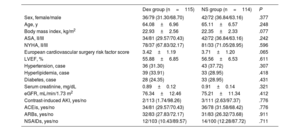
Baseline dataBaseline data indicated there were no substantial differences in age, sex, body mass index, American Society of Anesthesiologists grade, New York Heart Association grade, European cardiovascular surgery risk factor score, left ventricular ejection fraction, or complications due to hypertension, hyperlipidemia and diabetes among the 2 cohorts (P>.05). The use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs was also comparable between the 2 cohorts (P>.05) (table 1).
Comparison of general clinical data between the 2 groups
| Dex group (n=115) | NS group (n=114) | P | |
|---|---|---|---|
| Sex, female/male | 36/79 (31.30/68.70) | 42/72 (36.84/63.16) | .377 |
| Age, y | 64.08±6.96 | 65.11±6.57 | .248 |
| Body mass index, kg/m2 | 22.93±2.56 | 22.35±2.33 | .077 |
| ASA, II/III | 34/81 (29.57/70.43) | 42/72 (36.84/63.16) | .242 |
| NYHA, II/III | 78/37 (67.83/32.17) | 81/33 (71.05/28.95) | .596 |
| European cardiovascular surgery risk factor score | 3.42±1.19 | 3.71±1.20 | .065 |
| LVEF, % | 55.88±6.85 | 56.56±6.53 | .611 |
| Hypertension, case | 36 (31.30) | 43 (37.72) | .307 |
| Hyperlipidemia, case | 39 (33.91) | 33 (28.95) | .418 |
| Diabetes, case | 28 (24.35) | 33 (28.95) | .431 |
| Serum creatinine, mg/dL | 0.89±0.12 | 0.91±0.14 | .321 |
| eGFR, mL/min/1.73 m2 | 76.34±12.46 | 75.21±11.34 | .412 |
| Contrast-induced AKI, yes/no | 2/113 (1.74/98.26) | 3/111 (2.63/97.37) | .776 |
| ACEis, yes/no | 34/81 (29.57/70.43) | 36/78 (31.58/68.42) | .776 |
| ARBs, yes/no | 32/83 (27.83/72.17) | 31/83 (26.32/73.68) | .911 |
| NSAIDs, yes/no | 12/103 (10.43/89.57) | 14/100 (12.28/87.72) | .711 |
ACEis, angiotensin-converting enzyme inhibitors; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association; ARBs, angiotensin receptor blockers; Dex, dexmedetomidine; eGFR, estimated glomerular filtration rate; EuroSCORE, European Cardiac Surgery Risk Factor Score; LVEF, left ventricular ejection fraction; NS, normal saline; NSAIDs, nonsteroidal anti-inflammatory drugs.
The data are presented as No. (%) or mean±standard deviation.
Compared with the NS group.
P>.05, the difference was not statistically significant.
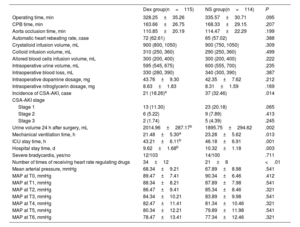
Among the 229 patients, 58 (25.33%) showed different degrees of CSA-AKI, of which 36 (15.72%) were identified as having AKI stage 1, 11 (6.55%) as AKI stage 2, and 7 (3.06%) as AKI stage 3. Within the Dex cohort, 21 patients (18.26%) developed CSA-AKI, of which 13 (11.30%) had AKI stage 1, 6 (5.22%) had AKI stage 2, and 2 (1.74%) had AKI stage 3. In the NS cohort, 37 patients (32.46%) developed CSA-AKI, including 23 (20.18%) with AKI stage 1, 9 (7.89%) with AKI stage 2, and 5 (4.39%) with AKI stage 3. The incidence of CSA-AKI was significantly lower in the Dex group than in the NS group (18.26% vs 32.46%, P=.014) (table 2).
Comparison of intraoperative and postoperative conditions between the 2 groups
| Dex group(n=115) | NS group(n=114) | P | |
|---|---|---|---|
| Operating time, min | 328.25±35.26 | 335.57±30.71 | .095 |
| CPB time, min | 163.66±26.75 | 168.33±29.15 | .207 |
| Aorta occlusion time, min | 110.85±20.19 | 114.47±22.29 | .199 |
| Automatic heart rebeating rate, case | 72 (62.61) | 65 (57.02) | .388 |
| Crystalloid infusion volume, mL | 900 (800, 1050) | 900 (750, 1050) | .309 |
| Colloid infusion volume, mL | 310 (250, 360) | 290 (250, 360) | .499 |
| Allored blood cells infusion volume, mL | 300 (200, 400) | 300 (200, 400) | .222 |
| Intraoperative urine volume, mL | 595 (545, 675) | 600 (555, 700) | .235 |
| Intraoperative blood loss, mL | 330 (280, 390) | 340 (300, 390) | .387 |
| Intraoperative dopamine dosage, mg | 43.76±9.30 | 42.35±7.62 | .212 |
| Intraoperative nitroglycerin dosage, mg | 8.63±1.83 | 8.31±1.59 | .169 |
| Incidence of CSA-AKI, case | 21 (18.26)a | 37 (32.46) | .014 |
| CSA-AKI stage | |||
| Stage 1 | 13 (11.30) | 23 (20.18) | .065 |
| Stage 2 | 6 (5.22) | 9 (7.89) | .413 |
| Stage 3 | 2 (1.74) | 5 (4.39) | .245 |
| Urine volume 24 h after surgery, mL | 2014.96±287.17b | 1895.75±294.62 | .002 |
| Mechanical ventilation time, h | 21.48±5.30a | 23.28±5.62 | .013 |
| ICU stay time, h | 43.21±6.11b | 46.18±6.91 | .001 |
| Hospital stay time, d | 9.62±1.68b | 10.32±1.18 | .003 |
| Severe bradycardia, yes/no | 12/103 | 14/100 | .711 |
| Number of times of receiving heart rate regulating drugs | 34±12 | 21±8 | <.01 |
| Mean arterial pressure, mmHg | 68.34±9.21 | 67.89±8.98 | .541 |
| MAP at T0, mmHg | 89.47±7.41 | 90.34±6.46 | .412 |
| MAP at T1, mmHg | 88.34±8.21 | 87.89±7.98 | .541 |
| MAP at T2, mmHg | 86.47±9.41 | 85.34±8.46 | .321 |
| MAP at T3, mmHg | 84.34±10.21 | 83.89±9.98 | .541 |
| MAP at T4, mmHg | 82.47±11.41 | 81.34±10.46 | .321 |
| MAP at T5, mmHg | 80.34±12.21 | 79.89±11.98 | .541 |
| MAP at T6, mmHg | 78.47±13.41 | 77.34±12.46 | .321 |
CPB, cardiopulmonary bypass; CSA-AKI, cardiac surgery-associated acute kidney injury; Dex, dexmedetomidine; ICU, intensive care unit; MAP, mean arterial pressure, NS, normal saline.
The data are expressed as No. (%) or mean±standard deviation
Compared with the NS group.
There were no marked differences in operating time, CPB time, occlusion time of the ascending aorta, automatic heart relapse rate, intraoperative fluid (crystal, colloid and allogeneic blood cell) infusion volume, intraoperative urine volume, intraoperative blood loss (total blood loss minus autotransfusion) and intraoperative dosages of dopamine and nitroglycerin between the 2 groups (P>.05). Compared with the value in the NS group, the volume of urine produced 24hours postsurgery in the Dex group was significantly elevated, the length of postoperative mechanical ventilation and intensive care unit residency was notably shorter, and the number of postoperative hospitalization days was statistically lower (P<.05). There was no marked variance between the 2 groups in the number of patients with severe bradycardia (P>.05). However, the frequency at which the patients in the Dex group were administered HR-modulating drugs was substantially higher than that of the patients in the NS group (P<.01). This implies that Dex can cause bradycardia, especially when an initial dose is administered, and that HR-modulating drugs may be necessary to prevent or treat this adverse effect. There was no appreciable difference in the mean arterial pressure between the 2 groups (P>.05) (table 2).
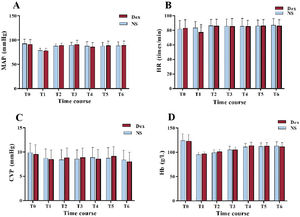
Perioperative hemodynamic changesThere was no appreciable difference in mean arterial pressure, central venous pressure and hemoglobin between the 2 cohorts at each time point (P>.05). Compared with the baseline value in the control group, HR at the initial time point in the Dex group was significantly reduced (P<.01). The data showed that although HR (83.47±8.41 times/min vs 77.34±6.46 times/min) was significantly lower at the end of surgery in the Dex group, it was still within the normal and safe range. However, in patients with coronary heart disease, this HR was more conducive to maintaining the balance of myocardial oxygen supply and demand, reducing myocardial energy consumption, alleviating myocardial injury and promoting myocardial protection than the HR of the patients in the NS group at this time point. This difference in HR can be attributed to the sedative and sympatholytic effects of Dex, which can lower the HR and reduce myocardial oxygen demand (figure 3).
Comparison of hemodynamic changes at each time between the 2 groups. A, mean arterial pressure (MAP) values; B, heart rate (HR) values; C, central venous pressure (CVP) values; and D, hemoglobin (Hb) values. Compared with the NS group, *P<.01. Dex, dexmedetomidine; NS, normal saline.
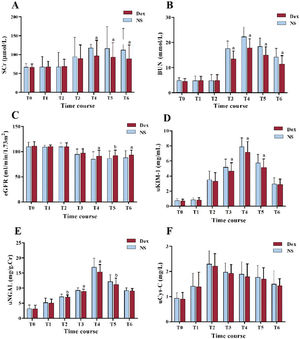
There were no significant differences in urinary cystatin C levels between the 2 cohorts at any time point (P>.05). Compared with the NS group, at T4–T6, the SCr concentration in the Dex group was substantially decreased, and the approximate rate of glomerular filtration was notably enhanced, with the eGFR value considerably increased (P<.05); at T3–T6, the blood urea nitrogen concentration in the Dex group was notably reduced (P<.01); at T3–T5, the urinary kidney damage molecule-1 protein concentration in the Dex group was markedly decreased (P<.01). At T2–T5, the concentration of urinary neutrophil gelatinase-associated lipocalcin in the Dex group was substantially lower (P<.05). These outcomes indicated that Dex significantly improved renal function in patients with CABG under CPB (figure 4).
Comparison of renal function between the 2 patient groups. A, serum creatinine (SCr) levels; B, serum blood urea nitrogen (BUN) levels; C, estimated glomerular filtration rate (eGFR) values; D, urine kidney injury molecule-1 (uKIM-1) levels; E, urine neutrophil gelatinase-associated lipocalin (NGAL) levels; and F, urine cystatin C (uCys-C) levels. Compared with the NS group, aP<.01; bP<.05. Dex, dexmedetomidine; NS, normal saline.
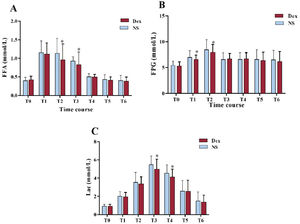
In contrast to the level in the NS group, the free fatty acid concentration in the Dex group was substantially decreased at T2–T3 (P<.01). At T1–T2, fasting plasma glucose status in the Dex group was notably lower (P<.01). At T3–T4, the lactate status in the Dex group was notably reduced (P<.01). These outcomes suggested that Dex maintained the balance of fatty acid and glucose metabolism and reduced lactic acid generation (figure 5).
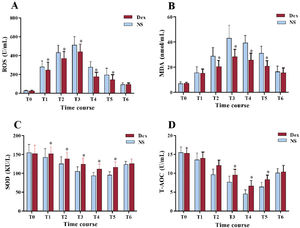
Oxidative stress levelsIn contrast to the measurements in the NS group, the reactive oxygen species status was significantly reduced in the Dex group, and superoxide dismutase activity was notably increased in the Dex group at T1–T5 (P<.01). At T2–T5, the malondialdehyde level in the Dex group was markedly reduced (P<.01). At T3–T5, the total antioxidant capacity status in the Dex group was significantly elevated (P<.01). The above results confirmed that Dex significantly reduced the oxidative stress reaction caused by surgery (figure 6).
Comparison of oxidative stress levels between the 2 groups. A, reactive oxygen species (ROS) levels; B, malondialdehyde (MDA) levels; C, superoxide dismutase (SOD) activity; and D, total antioxidant capacity (T-AOC) levels. Compared with the NS group, *P<.01. Dex, dexmedetomidine; NS, normal saline.
Globally, the increase in perioperative AKI, often requiring renal replacement therapy, is concerning, with severe AKI linked to an in-hospital mortality rate of more than 60%.5,25 Early prevention, appropriate intraoperative care, prompt diagnoses, and postoperative treatment are vital in managing AKI during surgery like CABG. Anaesthetic choice is crucial for renal protection.26 Ischemia/reperfusion in cardiac surgery, especially with CPB in CABG, significantly contributes to CSA-AKI. A retrospective study showed no significant difference in AKI between Dex and non-Dex groups (8% vs 7%; P=.333).27 In our study, the incidence of CSA-AKI dropped from 32.5% in the NS group to 18.3% with Dex, confirming its effectiveness in reducing CSA-AKI, balancing metabolism, and reducing oxidative stress.
In AKI diagnosis, SCr, blood urea nitrogen, eGFR, cystatin C, and urinary volume are key,28 but SCr and blood urea nitrogen have limitated sensitivity and specificity, potentially delaying interventions.29–34 Our study used sensitive markers such as urinary kidney damage molecule-1 and neutrophil gelatinase-associated lipocalcin, and found that Dex reduced their postsurgery increase. Renal tubular epithelial cells, which are vulnerable to oxygen and energy shortages during CABG with CPB, can undergo ischemia-reperfusion injury from imbalances in supply and demand, leading to sodium and calcium buildup and tissue acidosis.35–38 Dex was shown to modify serum free fatty acids, fasting plasma glucose, and lactic acid levels, which increased during surgery.
Cardiac surgery with CPB triggers inflammation and oxidative stress, elevating reactive oxygen species levels. Our findings indicate that Dex significantly reduced oxidative stress reactions caused by cardiac surgery.
This study found that most of the significant changes or benefits occurred at T3 (24hours after surgery) and not later (T4, T5). It is speculated that this is because T3 is the time at which the effects of Dex are most pronounced, as it has a half life of about 2hours and a duration of action of about 6hours. Therefore, at T3, Dex can still exert its protective effects on renal function, energy metabolism and oxidative stress, while at T4 and T5, the effects of Dex may have worn off, and the recovery process may have started.
LimitationsNevertheless, the present study has inherent limitations, including its single-center design and its limited sample size. Prolonged follow-up is crucial for a comprehensive understanding of the therapeutic implications of Dex. Future research should include more extensive study areas and populations with longer-term follow-ups to investigate in depth the mechanism through which Dex mitigates CSA-AKI in patients undergoing CABG with extracorporeal circulation.
CONCLUSIONSDex can reduce the incidence of CSA-AKI in patients undergoing CPB, improve renal function, maintain the balance of fatty acid and glucose metabolism, and reduce oxidative stress reactions; furthermore, it can shorten postoperative hospital stay. Dex may reduce renal ischemia-reperfusion injury by regulating fatty acid and glucose metabolism and reducing oxidative stress reactions.
FUNDINGThis study was supported by grants from the Health Research Program of Anhui Province (AHWJ2022b024), the Natural Science Key Foundation of the Education Department of Anhui Province (KJ2021A0712 and 2023AH051993), the Special Project of Clinical Medicine Research Transformation in Anhui Province (202304295107020082), and the Provincial Undergraduate Training Programs for Innovation and Entrepreneurship (S202310367144 and S202310367137).
ETHICAL CONSIDERATIONSThis study was conducted in accordance with the declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College (Approval No. 2021KY013). Written informed consent was obtained from all participants.
The datasets for this study are available from the corresponding author upon reasonable request. We defined sex as the biological characteristics of the participants and gender as the social and cultural factors that influence the participants’ behavior and identity.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tools or services to create or modify the content of this document were used.
AUTHORS’ CONTRIBUTIONSConception and design: C. Zhang and Y. Zhang. Administrative support: D. Liu and M. Mei. Provision of study materials or patients: N. Song and Q. Zhuang. Collection and assembly of data: Y. Jiang, Y. Guo and G. Liu. Data analysis and interpretation: X. Li, and L. Ren. Manuscript writing: all authors. Final approval of manuscript: all authors.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
Cardiac surgery-associated acute kidney injury (CSA-AKI) is a significant postoperative complication with high morbidity and mortality that is especially prevalent after procedures such as coronary artery bypass grafting (CABG) and heart valve replacement under extracorporeal circulation. The primary cause includes renal tubular damage from ischemia-reperfusion, imbalance in renal oxygen supply-demand, and inflammation linked to cardiopulmonary assistance. Current strategies for the prevention and mitigation of CSA-AKI are still under investigation, highlighting the need for effective interventions in this area.
WHAT DOES THIS STUDY ADD?This study demonstrates that dexmedetomidine (Dex), a selective α2-adrenoceptor agonist, significantly reduces the incidence of CSA-AKI in patients undergoing CABG with cardiopulmonary bypass. Dex not only improves renal function but also maintains the balance of fatty acid and glucose metabolism and reduces oxidative stress reactions. The findings suggest that Dex mitigates renal ischemia-reperfusion injury, offering a potentially effective approach for renal protection in cardiac surgery, thereby shortening postoperative hospital stays and improving patient outcomes.
The authors would like to thank colleagues in the Department of Cardiac Surgery, the Department of Anesthesiology, colleagues in the clinical research team, and postgraduate students for their strong support in the implementation of clinical research, case data collection, and data summary.