Galectin-3 (Gal-3) and carbohydrate antigen 125 (CA125) have been associated with adverse outcomes after transcatheter aortic valve implantation (TAVI). Experimental data have suggested a potential molecular interaction. Therefore, we assessed the association of Gal-3 and CA125 with prognosis after TAVI.
MethodsA total of 439 patients were enrolled. The primary endpoint was a composite of all-cause mortality or readmission for worsening heart failure after TAVI.
ResultsThe primary endpoint occurred in 16.4%. Gal-3 was dichotomized at ≥ 8.71 ng/mL into elevated and not elevated. Gal-3 was elevated in 31.9% and was associated with a higher risk of the primary endpoint (25% vs 12.4%, HR, 2.26; P<.001). After multivariable adjustment, the association of elevated Gal-3 with the primary endpoint was borderline significant (HR, 1.59; P=.068). CA125 was dichotomized at ≥ 18.4 U/mL, accordingly. CA125 was elevated in 51.9% and was also associated with a higher risk of the primary endpoint (25.4% vs 6.6%, HR, 4.20; P<.001). After multivariable adjustment, elevated CA125 (HR, 2.83; P=.001) remained independently associated with the primary endpoint. A differential prognostic effect of Gal-3 was found across CA125 status (P for interaction=.048). Elevated Gal-3 was associated with a higher risk of the primary endpoint when CA125 was elevated (38.8% vs 18.2%, HR, 2.02; P=.015) but lacked significance when CA125 was not elevated (6.6% vs 6.7%, HR, 1.16; P=.981).
ConclusionsIn patients undergoing TAVI, Gal-3 predicted adverse clinical outcomes only when CA125 was elevated.
Keywords
Several biomarkers have been investigated to assess prognosis in transcatheter aortic valve implantation (TAVI). Carbohydrate antigen 125 (CA125) is associated with disease severity in aortic stenosis1 and adverse clinical outcome after TAVI.2 Recently, it has been shown that its predictive value is superior to that of natriuretic peptides3 and offers incremental prognostic information beyond that obtained by the European System for Cardiac Operative Risk Evaluation (EuroSCORE).4 Emerging evidence has identified CA125 as a specific binding partner of soluble lectins, such as galectin-3 (Gal-3).5,6 Gal-3 is a ubiquitous lectin that contains a carbohydrate-recognition domain enabling specific interactions with glycosylated proteins through which various molecular signaling pathways are mediated.5 Both CA125 and Gal-3 seem to be involved in similar signaling pathways, such as the regulation of cell adhesion, cell proliferation, and inflammation.6–8 Gal-3 represents an emerging marker of myocardial fibrosis, hypertrophy, and inflammation.7,9,10 In heart failure, Gal-3 has been linked to disease severity and adverse clinical outcomes, in both acute and chronic heart failure (CHF).7,11,12 However, in TAVI, results from available studies regarding its prognostic value are conflicting.13,14
Based on these experimental data suggesting a potential molecular interaction between Gal-3 and CA125 and the conflicting results regarding the prognostic value of Gal-3 in patients with severe aortic stenosis undergoing TAVI, we hypothesized that the predictive value of Gal-3 might be influenced by CA125 levels. Therefore, we assessed the prognostic value of Gal-3 and CA125 and further investigated their potential interaction in a large contemporary TAVI cohort.
METHODSStudy Population and ProceduresThis study included 439 consecutive patients with symptomatic severe aortic stenosis undergoing transfemoral TAVI at the Department of Cardiovascular Disease, Deutsches Herzzentrum München, Munich, Germany between April 2015 and December 2016. The multidisciplinary heart team discussed all patients and consensus was reached on the therapeutic strategy in each patient. Biomarkers were determined from routine blood samples at least 24hours before TAVI. Gal-3 was measured using an immunoassay (Quantikine Human Galectin-3 Immunoassay; R&D Systems, Minneapolis, MN, USA) and CA125 was determined using an electrochemiluminescence immunoassay (Cobas e411 analyzer, Roche Diagnostics GmbH, Mannheim, Germany). All patients provided written informed consent.
Definition of Endpoints and Follow-upThe primary endpoint of this study was a composite of all-cause mortality or unplanned readmission for worsening CHF during follow-up. In addition, all-cause mortality and readmission for CHF were analyzed separately. All clinical endpoints, procedural data and in-hospital complications were categorized according to the updated Valve Academic Research Consortium-2 criteria.15 All data were prospectively collected during routine visits at the outpatient clinic, by referring to hospital documentation, contacting the primary care physician, or by direct contact with patients or their relatives.
Statistical AnalysisData distribution was tested for normality using the Shapiro-Wilks test. Categorical variables are expressed as frequencies and proportions and were compared using the chi-square or Fisher exact test, as appropriate. Continuous variables are presented as mean with standard deviation (SD) or median with interquartile range [IQR] and compared using the Student t-test or Mann-Whitney U-test, respectively.
For dichotomous analysis, 2 groups of patients with elevated and not elevated Gal-3 levels were identified by maximally selected rank statistics (R-package “survminer”, version 0.4.0). CA125 was dichotomized according to a previously published cutoff (≥ 18.4 U/mL) into elevated and not elevated.3 Event rates of the primary endpoint and separately all-cause mortality and readmission for CHF were calculated as crude rates. Cumulative events during the first 12 months after TAVI were visualized using the Kaplan-Meier method with differences tested using the log-rank test.
The independent association of elevated Gal-3 and CA125 values, modeled separately and together, with time to the primary endpoint, as well as all-cause mortality and CHF separately, was assessed using multivariable Cox proportional hazards regression analyses. The hazard ratios (HR) with their 95% confidence intervals (95%CI) were computed. For CHF, the subdistribution hazard ratios (Fine and Gray model)16 with death as a competing event were also calculated (R-package “cmprsk”, version 2.2-7). Covariates in the multivariable model were selected using the LASSO (Least Absolute Shrinkage and Selection Operator) regression method entering all baseline variables as candidates (R-package “glmnet”, version 2.0-13). The resulting variables were age, logistic EuroSCORE I, previous myocardial infarction, previous cancer, atrial fibrillation, mitral regurgitation grade III/IV, pulmonary hypertension (defined as pulmonary artery pressure > 60 mmHg), hemoglobin values, creatinine clearance, N-terminal pro-B-type natriuretic peptide, and mean transvalvular gradient. Additionally, left ventricular ejection fraction<35% and C-reactive protein values were included due to their known prognostic value and the findings in the univariate analyses. Missing baseline data (0.14%) were imputed by predictive mean matching (R-package “mice”, version 2.46).
The proportional hazard assumption of multivariable models for the primary endpoint containing Gal-3 (dichotomic), CA125 (dichotomic) and both variables (dichotomic) was checked using scaled Schoenfeld residuals. The assumption of proportional hazards was met, expressed by a nonsignificant relationship between global residual and time (global P values 0.121, 0.290 and 0.151, respectively).
When modeled together, the interaction of continuous and dichotomous Gal-3 values within CA125 strata (elevated vs not elevated CA125) was tested. The differential prognostic value of elevated Gal-3 regarding the primary endpoint according to CA125 status (elevated vs not elevated CA125) was further analyzed. The incremental prognostic usefulness of elevated Gal-3 to a model containing elevated CA125 and baseline variables, as well as within in each CA125 stratum, was evaluated by calculating the integrated discrimination improvement and the net reclassification improvement with the corresponding 95%CIs (Stata package “incrisk” version 1.0.5). These statistical indices have been developed to evaluate the added predictive value of a new marker to a base model and have been described in detail previously.17
A 2-sided P value < .05 was considered statistically significant. Statistical analyses were performed using R (version 3.3.2, R Foundation for Statistical Computing, Vienna, Austria) and Stata 14 (Stata Corp, TX, USA).
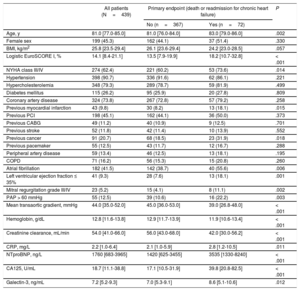
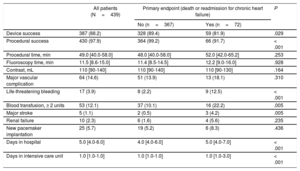
RESULTSPatient Characteristics and OutcomesBaseline and procedural characteristics of the entire patient population and according to the primary endpoint are displayed in Table 1 and Table 2. During a median follow-up of 371 days [219-402], the primary endpoint occurred in 16.4% (72 of 439) of the patients. Separately, all-cause mortality was 8.4% (37 of 439) and readmission for CHF occurred in 9.8% (43 of 439) of the patients. Patients experiencing the primary endpoint were older, had a higher predicted operative risk according to logistic EuroSCORE I, and were more symptomatic according to the New York Heart Association functional class. Procedural and in-hospital course was more complicated in these patients (Table 2).
Baseline Characteristics According to the Primary Endpoint
| All patients (N=439) | Primary endpoint (death or readmission for chronic heart failure) | P | ||
|---|---|---|---|---|
| No (n=367) | Yes (n=72) | |||
| Age, y | 81.0 [77.0-85.0] | 81.0 [76.0-84.0] | 83.0 [79.0-86.0] | .002 |
| Female sex | 199 (45.3) | 162 (44.1) | 37 (51.4) | .330 |
| BMI, kg/m2 | 25.8 [23.5-29.4] | 26.1 [23.6-29.4] | 24.2 [23.0-28.5] | .057 |
| Logistic EuroSCORE I, % | 14.1 [8.4-21.1] | 13.5 [7.9-19.9] | 18.2 [10.7-32.8] | < .001 |
| NYHA class III/IV | 274 (62.4) | 221 (60.2) | 53 (73.6) | .014 |
| Hypertension | 398 (90.7) | 336 (91.6) | 62 (86.1) | .221 |
| Hypercholesterolemia | 348 (79.3) | 289 (78.7) | 59 (81.9) | .499 |
| Diabetes mellitus | 115 (26.2) | 95 (25.9) | 20 (27.8) | .809 |
| Coronary artery disease | 324 (73.8) | 267 (72.8) | 57 (79.2) | .258 |
| Previous myocardial infarction | 43 (9.8) | 30 (8.2) | 13 (18.1) | .015 |
| Previous PCI | 198 (45.1) | 162 (44.1) | 36 (50.0) | .373 |
| Previous CABG | 49 (11.2) | 40 (10.9) | 9 (12.5) | .701 |
| Previous stroke | 52 (11.8) | 42 (11.4) | 10 (13.9) | .552 |
| Previous cancer | 91 (20.7) | 68 (18.5) | 23 (31.9) | .018 |
| Previous pacemaker | 55 (12.5) | 43 (11.7) | 12 (16.7) | .288 |
| Peripheral artery disease | 59 (13.4) | 46 (12.5) | 13 (18.1) | .195 |
| COPD | 71 (16.2) | 56 (15.3) | 15 (20.8) | .260 |
| Atrial fibrillation | 182 (41.5) | 142 (38.7) | 40 (55.6) | .006 |
| Left ventricular ejection fraction ≤ 35% | 41 (9.3) | 28 (7.6) | 13 (18.1) | .001 |
| Mitral regurgitation grade III/IV | 23 (5.2) | 15 (4.1) | 8 (11.1) | .002 |
| PAP > 60 mmHg | 55 (12.5) | 39 (10.6) | 16 (22.2) | .003 |
| Mean transaortic gradient, mmHg | 44.0 [35.0-52.0] | 45.0 [36.0-53.0] | 39.0 [26.8-48.0] | < .001 |
| Hemoglobin, g/dL | 12.8 [11.6-13.8] | 12.9 [11.7-13.9] | 11.9 [10.6-13.4] | < .001 |
| Creatinine clearance, mL/min | 54.0 [41.0-66.0] | 56.0 [43.0-68.0] | 42.0 [30.0-56.2] | < .001 |
| CRP, mg/L | 2.2 [1.0-6.4] | 2.1 [1.0-5.9] | 2.8 [1.2-10.5] | .011 |
| NTproBNP, ng/L | 1760 [683-3965] | 1420 [625-3455] | 3535 [1330-8240] | < .001 |
| CA125, U/mL | 18.7 [11.1-38.8] | 17.1 [10.5-31.9] | 39.8 [20.8-82.5] | < .001 |
| Galectin-3, ng/mL | 7.2 [5.2-9.3] | 7.0 [5.3-9.1] | 8.6 [5.1-10.6] | .012 |
BMI, body mass index; CA125, carbohydrate antigen 125; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; EuroSCORE, European System for Cardiac Operative Risk Evaluation; NTproBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PCI, percutaneous coronary intervention.
The data are expressed as No. (%) or median [interquartile range].
Procedural Characteristics and In-hospital Outcomes According to the Primary Endpoint
| All patients (N=439) | Primary endpoint (death or readmission for chronic heart failure) | P | ||
|---|---|---|---|---|
| No (n=367) | Yes (n=72) | |||
| Device success | 387 (88.2) | 328 (89.4) | 59 (81.9) | .029 |
| Procedural success | 430 (97.9) | 364 (99.2) | 66 (91.7) | < .001 |
| Procedural time, min | 49.0 [40.0-58.0] | 48.0 [40.0-58.0] | 52.0 [42.0-65.2] | .253 |
| Fluoroscopy time, min | 11.5 [8.6-15.0] | 11.4 [8.5-14.5] | 12.2 [9.0-16.0] | .928 |
| Contrast, mL | 110 [90-140] | 110 [90-140] | 110 [90-130] | .164 |
| Major vascular complication | 64 (14.6) | 51 (13.9) | 13 (18.1) | .310 |
| Life-threatening bleeding | 17 (3.9) | 8 (2.2) | 9 (12.5) | < .001 |
| Blood transfusion, ≥ 2 units | 53 (12.1) | 37 (10.1) | 16 (22.2) | .005 |
| Major stroke | 5 (1.1) | 2 (0.5) | 3 (4.2) | .005 |
| Renal failure | 10 (2.3) | 6 (1.6) | 4 (5.6) | .235 |
| New pacemaker implantation | 25 (5.7) | 19 (5.2) | 6 (8.3) | .436 |
| Days in hospital | 5.0 [4.0-6.0] | 4.0 [4.0-6.0] | 5.0 [4.0-7.0] | < .001 |
| Days in intensive care unit | 1.0 [1.0-1.0] | 1.0 [1.0-1.0] | 1.0 [1.0-3.0] | < .001 |
The data are expressed as No. (%) or median [interquartile range].
Median Gal-3 and CA125 levels were 7.2 ng/mL [5.2-9.3 ng/mL] and 18.7 U/mL [11.1-38.9 U/mL] with higher values in those experiencing the primary endpoint (8.6 ng/mL [5.1-10.6] vs 7.0 [5.2-9.1];P=.043) and (39.8 U/mL [20.7-82.7] vs 17.1 [10.5-32.0]; P=.001), respectively.
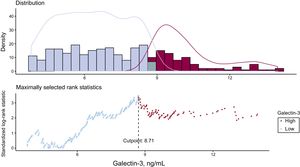
Prognostic Value of Galectin-3The ideal cutoff to predict the primary endpoint with Gal-3 was 8.71 ng/mL (Figure 1). Gal-3 values were elevated (≥ 8.71 ng/mL) in 31.9% (140/439) of the patients. Clinical and procedural characteristics and in-hospital outcomes of patients with elevated Gal-3 values are displayed in .
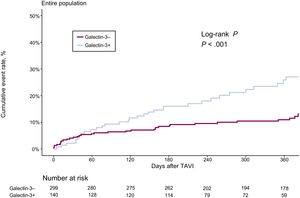
The cumulative event rate according to elevated Gal-3 and results from the univariate and multivariable analyses for the primary endpoint, as well as death and CHF separately, are provided in Table 3A. Elevated Gal-3 values were associated with a higher crude rate and risk of the primary endpoint (25% [35 of 140] vs 12.4% [37 of 299], HR, 2.26; 95%CI, 1.42-3.59; P=.001) in univariate analysis (Figure 2). After multivariable adjustment for baseline variables, the association of elevated Gal-3 with the primary endpoint was borderline significant (HR, 1.59; 95%CI, 0.97-2.62;P=.068) (Table 3A and Table 4).
Cumulative Event Rate and Multivariable Analysis for Galectin-3+ and CA125+
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Elevated galectin-3(≥ 8.71 ng/mL) | Crude event rate | Univariate analysis | Multivariable analysis | |||||
| No (n=299) | Yes (n=140) | HR (95%CI) | P | Harrel's C | HR (95%CI)a | P | Harrell's C | |
| Primary endpoint(death or readmission for CHF) | 37 (12.4) | 35 (25) | 2.26 (1.42-3.59) | < .001 | 0.586 | 1.59 (0.97-2.62) | .068 | 0.740 |
| Death | 19 (6.4) | 18 (12.9) | 2.12 (1.12-4.05) | .022 | 0.572 | 1.14 (0.56-2.35) | .717 | 0.766 |
| Readmission for CHF | 22 (7.4) | 21 (15) | 2.27 (1.25-4.13) | .007 | 0.594 | 1.65 (0.87-3.14) | .125 | 0.727 |
| 1.74 (0.93-3.25)b | .082 | |||||||
| B | ||||||||
|---|---|---|---|---|---|---|---|---|
| Elevated CA125(≥ 18.4 U/mL) | Crude event rate | Univariate analysis | Multivariable analysis | |||||
| No (n=211) | Yes (n=228) | HR (95%CI) | P | Harrel's C | HR (95%CI)a | P | Harrel's C | |
| Primary endpoint(death or readmission for CHF) | 14 (6.6) | 58 (25.4) | 4.20 (2.34-7.53) | < .001 | 0.664 | 2.83 (1.52-5.29) | .001 | 0.759 |
| Death | 8 (3.8) | 29 (12.7) | 3.39 (1.55-7.43) | .002 | 0.644 | 2.25 (0.96-5.29) | .062 | 0.772 |
| Readmission for CHF | 8 (3.8) | 35 (15.4) | 4.45 (2.06-9.60) | < .001 | 0.668 | 3.01 (1.32-6.84) | .008 | 0.755 |
| 2.87 (1.28-6.45)b | .011 | |||||||
95%CI, 95% confidence interval; CA125: carbohydrate antigen 125; CHF, chronic heart failure; CRP, C-reactive protein; EuroSCORE, European System for Cardiac Operative Risk Evaluation; HR: hazard ratio.
Unless otherwise indicated, data are expressed as No. (%).
Adjusted for age, logistic EuroSCORE I, previous myocardial infarction, previous cancer, atrial fibrillation, mitral regurgitation grade III/IV, pulmonary hypertension, hemoglobin, creatinine clearance, N-terminal pro-B-type natriuretic peptide, mean transvalvular gradient, left ventricular ejection fraction < 35% and CRP values.
Complete Results of Multivariable Analyses
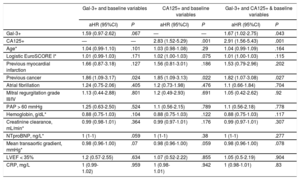
| Gal-3+ and baseline variables | CA125+ and baseline variables | Gal-3+ and CA125+ & baseline variables | ||||||
|---|---|---|---|---|---|---|---|---|
| aHR (95%CI) | P | aHR (95%CI) | P | aHR (95%CI) | P | |||
| Gal-3+ | 1.59 (0.97-2.62) | .067 | — | — | 1.67 (1.02-2.75) | .043 | ||
| CA125+ | — | — | 2.83 (1.52-5.29) | .001 | 2.91 (1.56-5.43) | .001 | ||
| Age* | 1.04 (0.99-1.10) | .101 | 1.03 (0.98-1.08) | .29 | 1.04 (0.99-1.09) | .164 | ||
| Logistic EuroSCORE I* | 1.01 (0.99-1.03) | .171 | 1.02 (1.00-1.03) | .075 | 1.01 (1.00-1.03) | .115 | ||
| Previous myocardial infarction | 1.66 (0.87-3.18) | .127 | 1.56 (0.81-3.01) | .186 | 1.53 (0.79-2.96) | .202 | ||
| Previous cancer | 1.86 (1.09-3.17) | .024 | 1.85 (1.09-3.13) | .022 | 1.82 (1.07-3.08) | .027 | ||
| Atrial fibrillation | 1.24 (0.75-2.06) | .405 | 1.2 (0.73-1.98) | .476 | 1.1 (0.66-1.84) | .704 | ||
| Mitral regurgitation grade III/IV | 1.13 (0.44-2.88) | .801 | 1.2 (0.49-2.93) | .691 | 1.05 (0.42-2.62) | .92 | ||
| PAP > 60 mmHg | 1.25 (0.63-2.50) | .524 | 1.1 (0.56-2.15) | .789 | 1.1 (0.56-2.18) | .778 | ||
| Hemoglobin, g/dL* | 0.88 (0.75-1.03) | .104 | 0.88 (0.75-1.03) | .122 | 0.88 (0.75-1.03) | .117 | ||
| Creatinine clearance, mL/min* | 0.99 (0.98-1.01) | .364 | 0.99 (0.97-1.01) | .176 | 0.99 (0.97-1.01) | .307 | ||
| NTproBNP, ng/L* | 1 (1-1) | .059 | 1 (1-1) | .38 | 1 (1-1) | .277 | ||
| Mean transaortic gradient, mmHg* | 0.98 (0.96-1.00) | .07 | 0.98 (0.96-1.00) | .059 | 0.98 (0.96-1.00) | .078 | ||
| LVEF < 35% | 1.2 (0.57-2.55) | .634 | 1.07 (0.52-2.22) | .855 | 1.05 (0.5-2.19) | .904 | ||
| CRP, mg/L | 1 (0.99-1.02) | .959 | 1 (0.98-1.01) | .942 | 1 (0.98-1.01) | .83 | ||
95%CI, 95% confidence interval; aHR, adjusted hazard ratio; CA125, carbohydrate antigen 125; CRP, C-reactive protein; Gal-3, galectin-3; LVEF, left ventricular ejection fraction; NTproBNP, N-terminal pro-B-type natriuretic peptide; PAP, pulmonary artery pressure.
Univariate and multivariable analyses for prediction of death and CHF are displayed in Table 3A. Elevated Gal-3 was associated with a significantly higher risk of death and CHF in univariate analysis, which, however, was lost after multivariable adjustment.
Prognostic Value of Carbohydrate Antigen 125CA125 values were elevated (≥ 18.4 U/mL) in 51.9% (228 of 439) of the patients. The cumulative event rate according to elevated CA125 and results from the univariate and multivariable analyses for the primary endpoint, as well as death and CHF separately, are provided in Table 3B. Elevated CA125 values were associated with a higher crude rate and risk of the primary endpoint (25.4% [58of 228] vs 6.6% [14 of 211], HR, 4.20; 95%CI, 2.34-7.53; P=.001) in the univariate analysis (). After multivariable adjustment, elevated CA125 (HR, 2.83; 95%CI, 1.52-5.29; P=.001) remained independently associated with the primary endpoint (Table 3B and Table 4). Uni- and multivariable analysis for separate prediction of death and CHF are displayed in Table 3B. Elevated CA125 independently predicted readmission for CHF but not all-cause mortality after multivariable adjustment.
Prognostic Value of Galectin-3 According to Levels of Carbohydrate Antigen 125Information on both biomarkers was modeled together in a multivariable model for predicting time to the primary endpoint adjusted for baseline variables. Elevated Gal-3 (HR, 1.67; 95%CI, 1.02-2.75; P=.043) and elevated CA125 values (HR, 2.91; 95%CI, 1.56-5.43; P=.001) were both independently associated with the primary endpoint (Table 4).
The interaction between Gal-3 values and CA125 stratae (elevated vs not elevated) was significant when Gal-3 was modeled as a continuous (0.049) and borderline significant as a dichotomous variable (0.115). This potential differential prognostic value of Gal-3 depending upon the state of CA125 was further analyzed.
When CA125 was not elevated, elevated Gal-3 was not associated with an increased rate and adjusted risk of the primary endpoint (6.6% [10 of 151] vs 6.7% [4 of 60], HR, 1.16; 95%CI, 0.28-3.74; P=0.981). However, when CA125 was elevated, elevated Gal-3 was associated with an increased rate and adjusted risk of the primary endpoint (38.8% [31 of 80] vs 18.2% [27 of 148], HR, 2.02; 95%CI, 1.15-3.55; P=.015], Figure 3 and Table 5). For death and readmission for CHF, there was also no difference in crude event rates for elevated Gal-3 when CA125 was not elevated, but crude event rates were almost twice as high when CA125 was elevated. After multivariable adjustment, this significant difference disappeared for mortality and was borderline significant for CHF (HR, 1.83; 95%CI, 0.89-3.77; P=.099, Table 5).
Crude Event Rates and Multivariable Analyses According to Gal-3+ Within Stratae of CA125+
| Crude event rate, No. (%) | Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Primary endpoint | HR (95%CI) | P | Harrel's C | HR (95%CI)a | P | Harrel's C | ||
| CA125– (n=211) | Gal-3– (n=151) | 10 (6.6) | 1.03 (0.32-3.29) | .959 | 0.484 | 1.16 (0.28-3.74) | .981 | 0.730 |
| Gal-3+ (n=60) | 4 (6.7) | |||||||
| CA125+ (n=228) | Gal-3– (n=148) | 27 (18.2) | 2.42 (1.44-4.07) | < .001 | 0.594 | 2.02 (1.15-3.55) | .015 | 0.738 |
| Gal-3+ (n=80) | 31 (38.8) | |||||||
| Death, No. (%) | HR (95%CI) | P | Harrel's C | HR (95%CI)a | P | Harrel's C | ||
|---|---|---|---|---|---|---|---|---|
| CA125- (n=211) | Gal-3– (n=151) | 6 (4.0) | 0.85 (0.17-4.21) | .841 | 0.554 | 0.89 (0.11-6.98) | .913 | 0.832 |
| Gal-3+ (n=60) | 2 (3.3) | |||||||
| CA125+ (n=228) | Gal-3– (n=148) | 13 (8.8) | 2.38 (1.15-4.96) | .020 | 0.592 | 1.76 (0.76-4.04) | .185 | 0.780 |
| Gal-3+ (n=80) | 16 (20.0) | |||||||
| Readmission for CHF, No. (%) | HR (95%CI) | P | Harrel's C | HR (95%CI)a | P | Harrel's C | ||
|---|---|---|---|---|---|---|---|---|
| CA125- (n=211) | Gal-3– (n=151) | 5 (3.3) | 1.55 (0.37-6.47) | .551 | 0.564 | 1.76 (0.36-8.63) | .488 | 0.714 |
| Gal-3+ (n=60) | 3 (5.0) | |||||||
| 1.79 (0.35-9.16)b | .480 | |||||||
| CA125+ (n=228) | Gal-3– (n=148) | 17 (11.5) | 2.22 (1.14-4.32) | .019 | 0.586 | 1.83 (0.89-3.77) | .099 | 0.730 |
| Gal-3+ (n=80) | 18 (22.5) | |||||||
| 1.87 (0.95-3.70)b | .072 |
95%CI, 95% confidence interval; +, elevated, –, not elevated; CA125, carbohydrate antigen 125; CHF, chronic heart failure; CRP, C-reactive protein; EuroSCORE, European System for Cardiac Operative Risk Evaluation; Gal-3, galectin-3; HR, hazard ratio.
Adjusted for age, logistic EuroSCORE I, previous myocardial infarction, previous cancer, atrial fibrillation, mitral regurgitation grade III/IV, pulmonary hypertension, hemoglobin, creatinine clearance, N-terminal pro-B-type natriuretic peptide, mean transvalvular gradient, left ventricular ejection fraction < 35% and CRP values.
When Gal-3 elevation was added to a model containing unelevated CA125 values and baseline variables, no improvement was found in the area under the curve of the model or in net reclassification improvement, or the integrated discrimination improvement. Only when CA125 was elevated, the addition of Gal-3 elevation provided incremental prognostic value ().
DISCUSSIONThe present study investigated the prognostic value of Gal-3 and the interaction between Gal-3 and CA125 for prognosis after TAVI. The negative prognostic value of Gal-3 was confirmed in a large contemporary cohort of TAVI patients. Additional analysis showed that this negative prognostic value was only present in the subgroup of patients with elevated CA125 values (≥ 18.4 U/mL), but did not reach statistical significance in those with lower CA125 levels. These findings confirm experimental and clinical data suggesting a potential molecular interaction between both biomarkers.
Biomarkers in Transcatheter Aortic Valve ImplantationTAVI is increasingly performed in patients with severe symptomatic aortic stenosis18 and its efficacy and safety in patients with high- and intermediate-risk for conventional aortic valve replacement have been demonstrated in large randomized controlled trials.19,20 Risk stratification in patients referred for TAVI is challenging and available risk scores, such as the EuroSCORE, have limited value and do not provide TAVI-specific variables.21,22 Several biomarkers have been tested for prognostic purposes2,13,23–25 and offer incremental value over and above established risk scores.4 Because several processes, such as inflammation, calcification, fibrosis/remodeling, and ventricular stress are involved in the pathophysiology of aortic valve stenosis, the incorporation of different biomarkers into clinical practice could potentially optimize risk stratification and the timing of the procedure.
Galectin-3 in Cardiovascular DiseaseGal-3 is an emerging biomarker in cardiovascular research. It is expressed in a variety of cells, including fibroblasts and leukocytes, and is a central mediator of several pathophysiological processes, including inflammation and fibrogenesis,26–28 which are major pathophysiological mechanisms operating in the failing heart.27 These effects are supported by clinical findings showing that higher levels of Gal-3 are related to severity of heart failure and adverse clinical outcomes, in both acute and chronic CHF.7,11,12,29
Clinical studies on Gal-3 in aortic stenosis are sparse.29,30 In these studies, Gal-3 levels did not correlate well with the severity of aortic valve stenosis and did not provide further prognostic information on the occurrence of adverse events.30 In TAVI, its prognostic value remains to be determined because the results from the available studies are conflicting.13,14 While Gal-3 independently predicted all-cause mortality in 1 study,13 its significance disappeared after multivariable adjustment in another.14
The conflicting results from available studies and the fact that baseline Gal-3 loses its significance after multivariable adjustment suggests an essential role of unidentified factors that warrants further investigations.
Carbohydrate Antigen 125 in Transcatheter Aortic Valve ImplantationElevated CA125 levels have been linked to disease severity in CHF due to aortic stenosis31–33 and are independently associated with adverse prognosis after TAVI.2 Recently, it has been shown that CA125 offers independent prognostic value beyond that of the EuroSCORE4 and is superior to natriuretic peptides,3 which are the only biomarkers that are acknowledged in current guidelines.34
Combination of Galectin-3 and Carbohydrate Antigen 125Recent experimental and clinical studies have suggested a potential molecular interaction between Gal-3 and CA125. Gal-3 contains a carbohydrate-recognition domain enabling specific interactions with glycosylated proteins, such as CA125.5,6 Both biomarkers seem to be involved in similar molecular signaling pathways, especially inflammatory responses,26,35 although their precise roles in cardiac inflammation have not been elucidated. Inflammation and fibrosis are key mechanisms operating in the failing heart.36
In acute heart failure, it has already been shown that the prognostic effect of Gal-3 depends on CA125 levels. Besides a mechanistic, molecular interaction, a possible heart failure-related inflammatory response for this association has been postulated.37 The biological activity of galectins is strongly dependent on the number of glycoconjugates.37 In prior experimental studies, CA125 was shown to be a galectin receptor counter.7,37 CA125, a heavily glycosylated protein, is upregulated in heart failure and aortic stenosis.1,2,32,37 Given the previous statements, we postulate that the Gal-3-CA125 interaction may represent a crucial factor in disease progression in TAVI patients. Elevation of both biomarkers probably identified a subset of patients with more adverse left ventricular remodeling in which TAVI procedures might have less impact on the natural course of the disease.
In this study, we confirm the prognostic value of CA125 in TAVI patients and additionally confirm prior data from heart failure patients suggesting a differential prognostic effect of Gal-3 across CA125 values.37 Interestingly, elevated Gal-3 levels were associated with an increased rate and adjusted risk of the primary endpoint only when CA125 values were elevated but lacked prognostic value when CA125 values were not elevated. Even more importantly, the incorporation of elevated Gal-3 to a risk model containing unelevated CA125 values did not improve the predictive capacity of this model.
Thus, Gal-3 alone does not seem to be an ideal biomarker for risk prediction in patients undergoing TAVI, as it mainly depends on CA125 levels. In TAVI patients, it appears reasonable to only determine Gal-3, if CA125 is elevated, since in this situation it offers optimal prognostic information.
Clinical ImplicationsCurrently, risk prediction and the optimal timing of TAVI procedures is insufficient. Biomarkers could potentially optimize risk prediction beyond conventional risk scores in TAVI patients. Recent evidence suggests improved outcomes in patients with severe aortic stenosis when there is early diagnosis and invasive treatment, even in lower-risk patients.38 The interaction of 2 biomarkers reflecting different pathophysiological pathways indicates the complexity of aortic stenosis pathophysiology and suggests an integration of a multimodal approach. CA125 seems to be a key player in this scenario and should be incorporated into clinical practice. In addition, further studies are warranted to explore the biological relevance of Gal-3-CA125 interaction as a potential therapeutic target.
LimitationsThis is an observational study conducted in a single center. The outcomes of patients undergoing interventional procedures is complex and multifactorial. Thus, potential additional clinical determinants and procedural factors may not have been recognized in this analysis.
CONCLUSIONSIn patients undergoing TAVI, Gal-3 predicts adverse clinical outcomes, but only in the presence of elevated CA125 values. These clinical findings confirm experimental data suggesting a potential molecular interaction between the 2 biomarkers. The clinical applicability of Gal-3 in addition to other biomarkers for risk stratification into clinical practice should be investigated in future prospective studies.
CONFLICTS OF INTERESTThis work was supported in part by grants from PIE15/00013. O. Husser and J. Núñez have received lecture fees from Roche. All other authors have reported no relationships relevant to the content of this manuscript.
- –
Several biomarkers have been investigated to assess prognosis in TAVI. A combination of biomarkers might be suitable due to the complexity of aortic stenosis. CA125 is associated with adverse prognosis after TAVI and offers incremental value over and above established risk scores. Gal-3 has been linked to disease severity and adverse events in heart failure. However, in TAVI, results regarding its prognostic value are controversial. Experimental studies have suggested a potential molecular interaction between both biomarkers.
- –
The present study investigated the individual prognostic value of Gal-3 and CA125 as well as the interaction between the 2 biomarkers with regard to prognosis after TAVI. The negative prognostic value of the 2 biomarkers, CA125 and Gal-3, was confirmed in a large contemporary cohort of TAVI patients. Additionally, this clinical study confirms data from experimental studies suggesting a molecular interaction between the 2 biomarkers. Analyses showed that the negative prognostic value of Gal-3 is only present in the subgroup of patients with elevated CA125 values. These data support the concept of integrating different biomarkers into clinical practice to further optimize risk prediction.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2018.09.006.