«Nothing is softer or more flexible than water, yet nothing can resist it»
—Lao Tzu
Periprocedural intravenous hydration and contrast media (CM) volume minimization are the standard of care to prevent contrast-associated acute kidney injury (CA-AKI).1 The purpose of hydration is to expand intravascular volume in order to increase renal perfusion, facilitate the transition of water-soluble CM through nephrons to reduce its cytotoxic impact, and ensure adequate diuresis before, during, and after CM administration. Although the European Society of Cardiology/European Association of Cardiothoracic Surgery Guidelines recommend hydration with normal saline at 1mL/kg/h (0.5mL/kg/h if left ventricular ejection fraction is ≤ 35% or New York Heart Association [NYHA] class is >2) from 12hours before to 24hours after CM exposure, several hydration protocols have been reported over the years (table 1).2,3 Most recently, the concept of guided-hydration regimens has been proposed to improve the safety and efficacy of CA-AKI preventive strategies. In particular, some tailored hydration regimens have been reported, according to: a) urine flow rate;4–8b) left ventricular end-diastolic pressure (LVEDP),9c) central venous pressure;10 and d) bioimpedance.11
Currently recommended hydration protocols with normal saline for preventing contrast-associated acute kidney injury
| Source | Volume | Duration* |
|---|---|---|
| American College of Radiology1 | 100 mL/h | 12 h before to 24 h after |
| European Society of Cardiology/European Association of Cardiothoracic Surgery Guidelines2 | 1 mL/kg/h(0.5 mL/kg/h if LVEF ≤ 35% or NYHA > 2) | 12 h before to 24 h after |
| Gupta et al.3 | 3 mL/h | 1 h before to h after |
LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class.
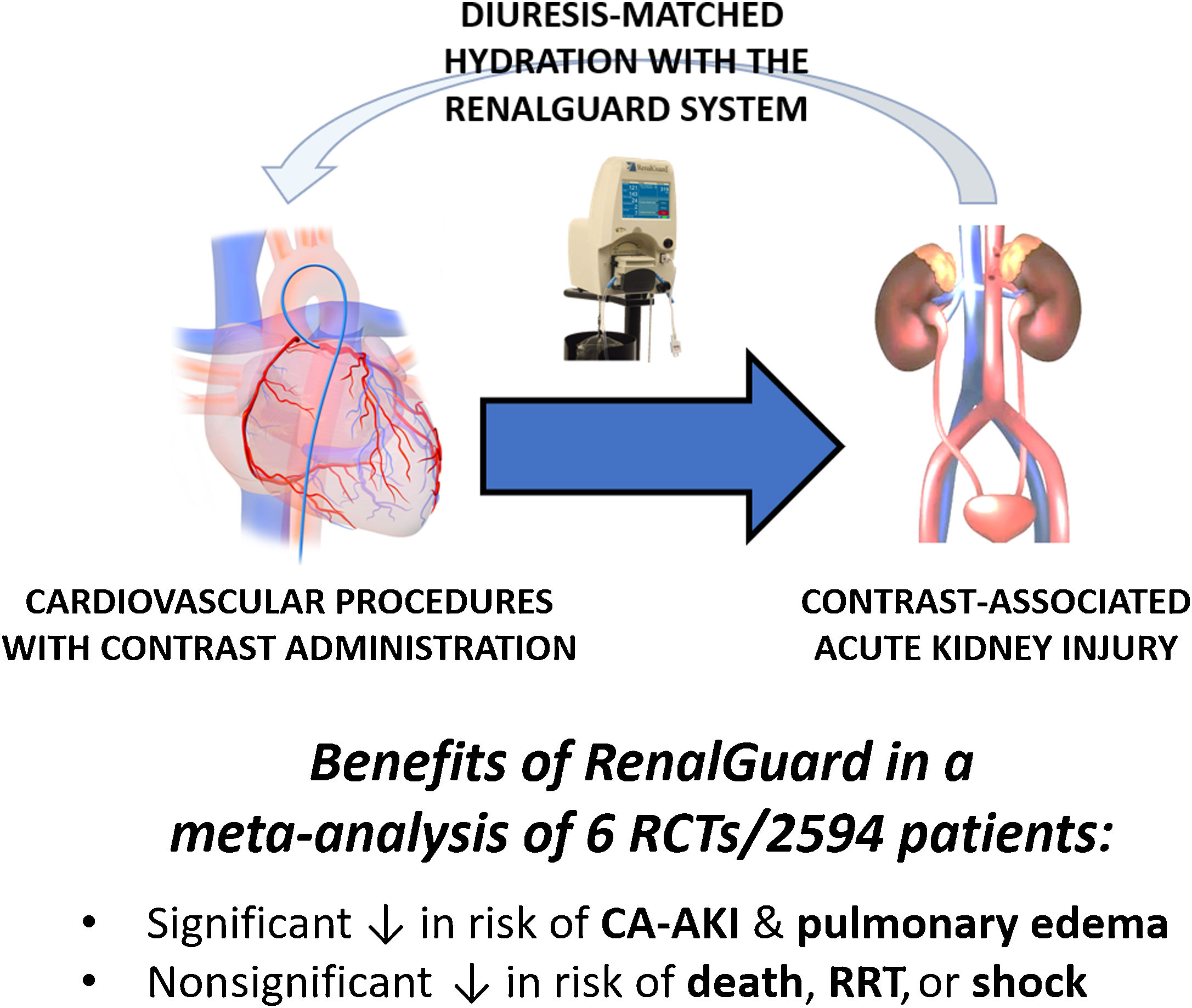
The main premise of these studies is that a high urine flow rate may reduce the incidence of CA-AKI through several effects. Notably, data from the Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation (PRINCE) study indicate that an increase in urine flow rate (≥ 150mL/h) reduces the toxic effect of CM.12 The RenalGuard system (PLC Medical System, Franklin, MA, United States) was designed to facilitate optimal hydration therapy (figure 1).13 This device allows high urine output to be achieved, while simultaneously balancing urine output and venous fluid infusion to prevent hypovolemia. Several randomized trials have demonstrated the effectiveness of this system in significantly reducing the incidence of CA-AKI compared with standard hydration in patients at high risk.4-8 Among them, the REMEDIAL III trial, which enrolled 702 patients at high risk for CA-AKI, has clearly demonstrated that urine flow rate-guided hydration carried out with the RenalGuard system is superior to LVEDP-guided hydration to prevent the composite of CA-AKI and/or acute pulmonary edema (relative risk [RR], 0.56; 95% confidence interval, 0.39-0.79; P = .036).14
In a recent article published in Revista Española de Cardiología, Occhipinti et al. report a Bayesian meta-analysis further supporting the clinical role of RenalGuard, showing that its use is associated with a lower risk of CA-AKI and acute pulmonary edema, with no differences in all-cause death, cardiogenic shock, or acute renal failure requiring renal replacement therapy compared with standard periprocedural intravenous hydration.15 Using a Bayesian approach, Occhipinti et al. were able to show a 95% probability of the RenalGuard system being superior to the control arm in reducing the risk of CA-AKI. Importantly, this outcome was obtained while limiting and even improving the potential consequences of uncontrolled hydration strategies (ie, volume overload and acute pulmonary edema). Furthermore, the treatment effect was even higher after exclusion of trials focusing on transcatheter aortic valve implantation, in which factors other than CM (such as hemodynamic conditions) may play a major role in the pathophysiology of CA-AKI.
This meta-analysis is timely and clinically relevant. First, this work highlights the notion that no standard hydration protocol exists, as shown in table 2, which details the different hydration protocols used in the control groups of the pooled trials. Second, as stated by Occhipinti et al., the RenalGuard system has been tested in only a few small randomized trials with low power to detect differences in clinical endpoints and high variability in patient characteristics and procedures. This may be the case of the recent Study Evaluating the Use of RenalGuard to Protect Patients at High Risk of AKI (STRENGTH).18 This randomized, multicenter, international, open-label, postmarket, prospective trial monitored by the Cardiovascular European Research Center, based in Massy, France, enrolled 250 participants with moderate to severe chronic kidney disease (estimated glomerular filtration rate, 15-40mL/min/m2) requiring a complex coronary, structural, or peripheral procedure with an expected contrast injection of at least 3 times the estimated glomerular filtration rate and randomized to either hydration with forced-balanced diuresis as provided by the RenalGuard or conventional intravenous saline hydration according to current guidelines. The primary endpoint, CA-AKI, occurred with similar frequency (15.9% in the RenalGuard group vs 13.9% in the control group; P = .6). The finding of this study differs from those of previous publications on the same topic, which showed that the RenalGuard device was beneficial in protecting against CA-AKI. A possible explanation is that this study was largely underpowered. Indeed, the study was powered at 0.9, with an alpha of 0.05 and each group required 133 patients per group to demonstrate a CA-AKI reduction from 25% to 10% with the RenalGuard device. However, only 60.3% (73 patients) in the study group and 50.8% (62 patients) in the control group met these inclusion criteria. Using the same alpha and CA-AKI reduction rates as mentioned above, this equates to the study being powered at 0.55, that is, it was significantly underpowered. Another outstanding caveat is that some very high-risk patients, such as those not achieving an adequate urine output (eg > 150mL/h) while under RenalGuard treatment, cannot benefit from this intervention.
Hydration protocols with normal saline for preventing contrast-associated acute kidney injury in the control groups of the trials included into the meta-analysis by Occhipinti et al.15
| Study | Volume | Duration* |
|---|---|---|
| CINEMA16 | 1-1.5 mL/kg/h | 12 h before to 12 h after |
| MYTHOS7 | 1 mL/kg/h | 12 h before to 12 h after |
| PROTECT TAVI6 | 1 mL/kg/h(0.5 mL/kg/h if LVEF < 30%) | 12 h before to 6 h after |
| REDUCE-AKI17 | 0.5-1 mL/kg/h | Intraprocedural to 6 h after |
| REMEDIAL III14 | 5 mL/h with LVEDP < 13 mmHg3 mL/h with LVEDP 13-18 mmHg1.5 mL/h with LVEDP > 18 mmHg | 1 h before to 6 h after |
| STRENGTH18 | 1 mL/kg/h(0.5 mL/kg/h if LVEF ≤ 35% or NYHA > 2) | 12 h before to 24 h after |
LVEDP, left ventricular end-diastolic pressure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class.
In conclusion, and in light of all the evidence on RenalGuard to date, including the timely and comprehensive meta-analysis by Occhipinti et al., 15 additional large randomized controlled studies are needed to clarify whether RenalGuard therapy should be recommended in all patients at risk or only in those at higher risk,19 according to the proposed CA-AKI risk scores.20,21 Another outstanding issue is whether RenalGuard is cost-effective in all instances or only in patients at higher baseline risk.3,17
FundingNone.
DisclosureG. Biondi-Zoccai has consulted for Amarin, Balmed, Cardionovum, Crannmedical, Endocore Lab, Eukon, Innovheart, Guidotti, Meditrial, Microport, Opsens Medical, Replycare, Teleflex, Terumo, and Translumina. All other authors report no conflicts of interest.