Contrast-associated acute kidney injury (CA-AKI) is a potential complication of procedures requiring administration of iodinated contrast medium. RenalGuard, which provides real-time matching of intravenous hydration with furosemide-induced diuresis, is an alternative to standard periprocedural hydration strategies. The evidence on RenalGuard in patients undergoing percutaneous cardiovascular procedures is sparse. We used a Bayesian framework to perform a meta-analysis of RenalGuard as a CA-AKI preventive strategy.
MethodsWe searched Medline, Cochrane Library and Web of Science for randomized trials of RenalGuard vs standard periprocedural hydration strategies. The primary outcome was CA-AKI. Secondary outcomes were all-cause death, cardiogenic shock, acute pulmonary edema, and renal failure requiring renal replacement therapy. A Bayesian random-effect risk ratio (RR) with corresponding 95% credibility interval (95%CrI) was calculated for each outcome. PROSPERO database number CRD42022378489.
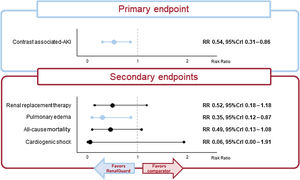
ResultsSix studies were included. RenalGuard was associated with a significant relative reduction in CA-AKI (median RR, 0.54; 95%CrI, 0.31-0.86) and acute pulmonary edema (median RR, 0.35; 95%CrI, 0.12-0.87). No significant differences were observed for the other secondary endpoints [all-cause death (RR, 0.49; 95%CrI, 0.13-1.08), cardiogenic shock (RR, 0.06; 95%CrI, 0.00-1.91), and renal replacement therapy (RR, 0.52; 95%CrI, 0.18-1.18)]. The Bayesian analysis also showed that RenalGuard had a high probability of ranking first for all the secondary outcomes. These results were consistent in multiple sensitivity analyses.
ConclusionsIn patients undergoing percutaneous cardiovascular procedures, RenalGuard was associated with a reduced risk of CA-AKI and acute pulmonary edema compared with standard periprocedural hydration strategies.
Keywords
Contrast-associated acute kidney injury (CA-AKI) is a complication of procedures requiring intravascular administration of iodinated contrast media1–3 and its risk depends on patient- and procedure-related factors.4 The strongest independent patient- and procedure-related predictors of CA-AKI are chronic kidney disease and use of high volumes of contrast medium, respectively.5–8 Strategies for CA-AKI prevention so far have focused on intravascular volume expansion (eg, by periprocedural use of isotonic saline, half-isotonic saline, isotonic sodium bicarbonate), pharmaceutical agents (eg, acetylcysteine, statins), and renal replacement therapies. Although several observational studies and randomized controlled trials have been published, there is no robust evidence of benefit with these interventions.9–13 Current guidelines recommend administering periprocedural intravenous fluids and minimizing the amount of contrast medium as the primary interventions to mitigate the risk of CA-AKI.14
RenalGuard (PLC Medical Systems, United States) is a system engineered to ensure adequate hydration while providing contrast medium clearance; the system delivers real-time isotonic intravenous hydration matched with high urine output induced by furosemide in the attempt to prevent conditions of either hyper- or hypovolemia.15 To date, RenalGuard has been tested in a few small randomized trials with low power to detect differences in major clinical endpoints and high variability in patient characteristics and procedures.16–21 Meta-analyses of RenalGuard are available but their conclusions are limited by the inclusion of observational studies. In addition, these meta-analyses mostly used frequentist models, which are limited when accounting for between-trial variability or if there are low event rates.22–24 Finally, a new trial of RenalGuard has been made available that was not included in the previous meta-analyses.21
Against this background, we used a Bayesian framework to perform a meta-analysis of RenalGuard as a CA-AKI preventive strategy for patients undergoing percutaneous cardiovascular procedures to a) increase the statistical power of individual trials for assessing infrequent outcomes; b) avoid the methodological limitations of previous frequentist meta-analyses; and c) update prior meta-analyses based on the most recent evidence available.
METHODSSearch strategy, selection criteria and data collectionThis meta-analysis was performed in compliance with the recommendations of the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) ()25; its protocol is registered in the Prospective Register of Systematic Reviews (PROSPERO) database (CRD42022378489). Eligible studies were included in the analysis if they: a) had a randomized design, irrespective of publication status and language; b) included ≥ 100 patients; c) enrolled adult patients aged ≥ 18 years undergoing percutaneous cardiovascular interventions; d) compared real-time matching of isotonic intravenous hydration with furosemide-induced diuresis by means of RenalGuard vs standard periprocedural hydration by means of intravenous saline solution; e) reported at least 1 of the prespecified outcomes of interest.
We conducted a systematic digital search in the Medline (via PubMed), Cochrane, and Web of Science databases from their inception to November 15, 2022. Search terms included “percutaneous cardiovascular intervention”, “coronary angiography”, “percutaneous coronary intervention”, “percutaneous left atrial appendage closure”, “transcatheter aortic valve implantation”, “contrast induced acute kidney injury”, “acute kidney injury”, “acute renal failure”, “contrast induced nephropathy”, “RenalGuard” and their combinations. Additional details on the search strategy are reported in . Two investigators (GO, MS) independently performed the literature search, and their findings were merged. After removal of duplicates, a title- and abstract-level selection was performed, followed by full-text screening of articles and available supplementary data (CL, GO, MS) (). Disagreements were solved by consensus with a senior author. Data from the eligible studies were extracted and tabulated, and the incidence rates of the endpoints of interest were collected from both the intervention and control arms.
Risk of bias assessmentThe risk of bias of each trial was independently assessed by 2 investigators (GO, CL) using the Cochrane Collaboration Risk-of-Bias-tool 2 (RoB 2) scale (). The publication bias was assessed by means of Egger's regression tests and by visual inspection of funnel plots.
OutcomesThe primary outcome was CA-AKI. Secondary outcomes were CA-AKI requiring renal replacement therapy, all-cause death, cardiogenic shock, and acute pulmonary edema. All the outcomes of the meta-analysis were reported as per their respective study definitions and were analyzed at the longest available follow-up.
Statistical analysisA pairwise meta-analysis of the study outcomes was first conducted using a Bayesian arm-level random-effect model. The likelihoods of the model were computed using a Markov-chain Monte Carlo simulation running 4 chains with overdispersed initial values. Gibbs’ sampling was based on 50 000 imputations after discarding 7000 iterations (burn-in period), and large uninformative prior distributions were used to impute posterior distributions. The posterior inference was summarized as median posterior risk ratio (RR) and 95% credibility interval (95%CrI). The probability of RenalGuard reducing the number of events compared with controls was also calculated using the cumulative posterior probability, expressed as a percentage. Chain convergence was checked by both graphical assessment and evaluation of Gelman-Rubin statistics, assuming convergence if the latter was <1.2. Heterogeneity was calculated as I2 and was categorized as low (< 30%), moderate (30-50%) or high (> 50%).
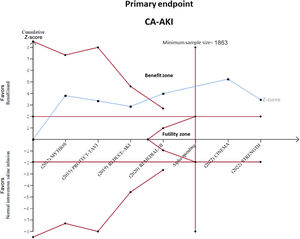
Precision, uncertainty and conclusiveness of the current evidence was also investigated by means of trial sequential analyses.26 Such analysis allows calculation of the required sample size needed to define a result as “conclusive” or not (ie, based on the number of events observed in the trials and cumulative relative risk reduction of the meta-analysis adjusted to the between-trial heterogeneity), and to adjust for the risk of type I error due to repeated significance testing, showing the relationship between the Z-curve of cumulative evidence and a set of benefit or futility boundaries.
We further investigated potential sources of heterogeneity by performing a random effects multivariate meta-analysis including the prespecified endpoints as dependent variables, and several post hoc subgroup analyses, including a leave-one-out analysis and analyses restricted to: a) studies of patients with a baseline eGFR<60mL/min/1.73m2, b) studies of patients who did or did not undergo complex procedures, c) studies reporting the Kidney Disease: Improving Global Outcomes (KDIGO) definition of CA-AKI, and d) studies including only elective procedures. Finally, we performed a random-effects Bayesian meta-regression analysis of the primary endpoint investigating the role of age, baseline ejection fraction, baseline glomerular filtration rate, diuretic use at baseline, and history of statin use at baseline.
All statistical analyses were conducted with JAGS version 4.3.0, R version 4.0.5 (R Foundation for Statistical Computing) using the packages metaphor, mvmeta and R2jags, and TSA (Trial Sequential Analysis) software version 9.0.5.10 beta.
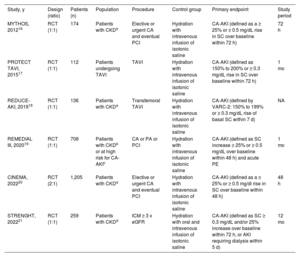
RESULTSStudy selection, characteristics, and risk of biasAfter data merging from independent searches and removal of duplicates, we identified a total of 261 potentially eligible articles. The search results of each database are reported in . After screening at the title, abstract and full-text levels, 6 studies (n=2590 patients) matched the criteria for inclusion in the meta-analysis and were appraised for quality (). The main features of the included trials are summarized in table 1. Briefly, 2 studies included only patients undergoing elective or urgent percutaneous coronary intervention16,20 and 1 also included patients undergoing peripheral interventions19; 1 study enrolled patients undergoing complex coronary, peripheral or structural interventions,21 and 2 studies included only patients undergoing transcatheter aortic valve implantation.17,18 The definition of CA-AKI varied slightly across studies, with cutoffs for serum creatinine ranging from ≥ 0.3 to ≥ 0.5mg/dL, and measurements within 72hours in 3 trials,16,17,21 within 48hours in 2 trials,19,20 and within 7 days in 1 trial.18 All 6 studies were at low risk for bias based on the RoB-2 evaluation tool ().
Main features of the included controlled trials
| Study, y | Design (ratio) | Patients (n) | Population | Procedure | Control group | Primary endpoint | Study period |
|---|---|---|---|---|---|---|---|
| MYTHOS, 201216 | RCT (1:1) | 174 | Patients with CKDa | Elective or urgent CA and eventual PCI | Hydration with intravenous infusion of isotonic saline | CA-AKI (defined as a ≥ 25% or ≥ 0.5 mg/dL rise in SC over baseline within 72 h) | 72 h |
| PROTECT TAVI, 201517 | RCT (1:1) | 112 | Patients undergoing TAVI | TAVI | Hydration with intravenous infusion of isotonic saline | CA-AKI (defined as 150% to 200% or ≥ 0.3 mg/dL rise in SC over baseline within 72 h) | 1 mo |
| REDUCE-AKI, 201918 | RCT (1:1) | 136 | Patients with CKDa | Transfemoral TAVI | Hydration with intravenous infusion of isotonic saline | CA-AKI (defined by VARC-2: 150% to 199% or ≥ 0.3 mg/dL rise of basal SC within 7 d) | NA |
| REMEDIAL III, 202019 | RCT (1:1) | 708 | Patients with CKDb or at high risk for CA-AKIc | CA or PA or PCI | Hydration with intravenous infusion of isotonic saline | CA-AKI (defined as SC increase ≥ 25% or ≥ 0.5 mg/dL over baseline within 48 h) and acute PE | 1 mo |
| CINEMA, 202220 | RCT (2:1) | 1,205 | Patients with CKDa | Elective or urgent CA and eventual PCI | Hydration with intravenous infusion of isotonic saline | CA-AKI (defined as a ≥ 25% or ≥ 0.5 mg/dl rise in SC over baseline within 48 h) | 48 h |
| STRENGHT, 202221 | RCT (1:1) | 259 | Patients with CKDd | ICM ≥ 3 x eGFR | Hydration with oral and intravenous infusion of isotonic saline | CA-AKI (defined as SC ≥ 0.3 mg/dL and/or 25% increase over baseline within 72 h, or AKI requiring dialysis within 5 d) | 12 mo |
CA, coronary angiography; CA-AKI, contrast-associated acute kidney injury; CKD, chronic kidney disease; ICM, iodinated contrast media; eGFR, estimated glomerular filtration rate; NA, not available; PA, peripheral angiography; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; SC, serum creatinine; TAVI, transcatheter aortic valve implantation.
RenalGuard was associated with a 46% significant reduction in the primary outcome of CA-AKI compared with standard periprocedural saline infusion (median RR, 0.54; 95%CrI, 0.31-0.86), displaying a 99.9% probability of reducing the risk of CA-AKI (figure 1). Moderate heterogeneity was observed (I2=39%). In the trial sequential analysis, the Z-curve for RenalGuard lay in the area of benefit and crossed the required information size, therefore suggesting that the current evidence for CA-AKI is conclusive (figure 2).
No statistically significant differences between RenalGuard and control were observed in the risk of renal replacement therapy (median RR, 0.52; 95%CrI, 0.18-1.18), all-cause death (median RR, 0.49; 95%CrI, 0.13-1.08) or cardiogenic shock (median RR, 0.06; 95%CrI, 0.00-1.91) (figure 1). Conversely, RenalGuard was associated with a 65% significantly lower risk of acute pulmonary edema (median RR, 0.35; 95%CrI, 0.12-0.87). RenalGuard had a high probability of ranking first with respect to all the secondary outcomes (95.2% for renal replacement therapy, 96.6% for all-cause death, 82.3% for cardiogenic shock, and 98.5% for pulmonary edema, respectively). No heterogeneity was found in any of the secondary outcomes (I2=0%). In the trial sequential analysis of renal replacement therapy and all-cause mortality, the required information size was not reached; however, the Z-curve of all-cause mortality entered the futility zone. Conversely, the Z-curve for pulmonary edema landed in the benefit zone and exceeded the minimum number of patients required to support this result. Since only 2 trials included cardiogenic shock as an endpoint and there were no events in either, it was not possible to calculate the sample size required to find a significant difference. Figure 3 shows the results of the trial sequential analysis for the other secondary endpoints.
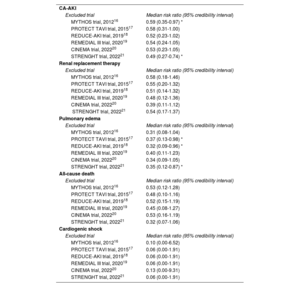
Sensitivity analysesTable 2 displays the results of the sensitivity analyses for the study outcomes. In all the analyses, the treatment effect of RenalGuard on the primary endpoint was directionally concordant with the results of the main analysis. Similar results were also found for all the other endpoints. The leave-one-out analysis identified potential sources of heterogeneity (table 3). In particular, the effect-size for CA-AKI was larger after removal of the STRENGHT trial (median RR, 0.49; 95%CrI, 0.27-0.74). Removal of any other trial except MYTHOS resulted in a loss of statistical significance. With respect to pulmonary edema, similar results were observed after removal of PROTECT TAVI and REDUCE-AKI (median RR, 0.37; 95%CrI, 0.13-0.98 and median RR, 0.32; 95%CrI, 0.09-0.96, respectively), whereas the removal of any other trial resulted in a loss of statistical significance. No sources of confounding were detected in any of the other endpoints. Conversely, the meta-regression and the multivariate meta-analysis showed no significant sources of heterogeneity among the covariates analyzed ().
Sensitivity analyses of prespecified outcomes
| Frequentist analysis | |
| Outcome | Risk ratio (95% credibility interval) |
| CA-AKI | 0.58 (0.42-0.79) * |
| Renal replacement therapy | 0.74 (0.37-1.48) |
| Pulmonary edema | 0.46 (0.23-0.91) * |
| All-cause death | 0.69 (0.42-1.15) |
| Cardiogenic shock | 0.70 (0.04-11.10) |
| Multivariate meta-analysis | |
| Outcome | Median risk ratio (95% credibility interval) |
| CA-AKI | 0.59 (0.45-0.77)* |
| Renal replacement therapy | 0.74 (0.37-1.48) |
| Pulmonary edema | 0.47 (0.25-0.87)* |
| All-cause death | 0.64 (0.38-1.10) |
| Cardiogenic shock | 0.70 (0.14-3.44) |
| Patients not undergoing complex procedures | |
| Outcome | Median risk ratio (95% credibility interval) |
| CA-AKI | 0.57 (0.30-1.11) |
| Renal replacement therapy | 0.68 (0.20-2.26) |
| Pulmonary edema | 0.34 (0.09-1.32) |
| All-cause death | 0.30 (0.03-1.65) |
| Cardiogenic shock | 0.10 (0.00-6.52) |
| Patients undergoing complex procedures | |
| Outcome | Median risk ratio (95% credibility interval) |
| CA-AKI | 0.46 (0.11-1.82) |
| Renal replacement therapy | 0.23 (0.03-1.42) |
| Pulmonary edema | 0.36 (0.04-2.54) |
| All-cause death | 0.57 (0.12-1.98) |
| Cardiogenic shock | 0.13 (0.00-9.21) |
| Limiting assessment of CA-AKI to 48hours after procedure | |
| Outcome | Median risk ratio (95% credibility interval) |
| CA-AKI | 0.56 (0.16-2.03) |
| Renal replacement therapy | 0.75 (0.15-3.73) |
| Pulmonary edema | 0.36 (0.04-2.54) |
| All-cause death | 0.37 (0.04-2.57) |
| Cardiogenic shock | 0.10 (0.00-6.52) |
| Patients undergoing elective procedures | |
| Outcome | Median risk ratio (95% credibility interval) |
| CA-AKI | 0.60 (0.25-1.34) |
| Renal replacement therapy | 0.44 (0.10-1.50) |
| Pulmonary edema | 0.28 (0.04-1.38) |
| All-cause death | 0.58 (0.16-1.58) |
| Cardiogenic shock | 0.06 (0.00-1.91) |
| Trials with CA-AKI definitions requiring a creatinine increase of> 0.5 mg/dL | |
| Outcome | Median risk ratio (95% credibility interval) |
| CA-AKI | 0.48 (0.16-1.23) |
| Renal replacement therapy | 0.60 (0.14-2.10) |
| Pulmonary edema | 0.28 (0.04-1.38) |
| All-cause death | 0.32 (0.05-1.43) |
| Cardiogenic shock | 0.06 (0.00-1.91) |
CA-AKI, contrast-associated acute kidney injury.
Leave-one-out analysis
| CA-AKI | |
| Excluded trial | Median risk ratio (95% credibility interval) |
| MYTHOS trial, 201216 | 0.59 (0.35-0.97) * |
| PROTECT TAVI trial, 201517 | 0.58 (0.31-1.00) |
| REDUCE-AKI trial, 201918 | 0.52 (0.23-1.02) |
| REMEDIAL III trial, 202019 | 0.54 (0.24-1.05) |
| CINEMA trial, 202220 | 0.53 (0.23-1.05) |
| STRENGHT trial, 202221 | 0.49 (0.27-0.74) * |
| Renal replacement therapy | |
| Excluded trial | Median risk ratio (95% credibility interval) |
| MYTHOS trial, 201216 | 0.58 (0.18-1.46) |
| PROTECT TAVI trial, 201517 | 0.55 (0.20-1.32) |
| REDUCE-AKI trial, 201918 | 0.51 (0.14-1.32) |
| REMEDIAL III trial, 202019 | 0.48 (0.12-1.36) |
| CINEMA trial, 202220 | 0.39 (0.11-1.12) |
| STRENGHT trial, 202221 | 0.54 (0.17-1.37) |
| Pulmonary edema | |
| Excluded trial | Median risk ratio (95% credibility interval) |
| MYTHOS trial, 201216 | 0.31 (0.08-1.04) |
| PROTECT TAVI trial, 201517 | 0.37 (0.13-0.98) * |
| REDUCE-AKI trial, 201918 | 0.32 (0.09-0.96) * |
| REMEDIAL III trial, 202019 | 0.40 (0.11-1.23) |
| CINEMA trial, 202220 | 0.34 (0.09-1.05) |
| STRENGHT trial, 202221 | 0.35 (0.12-0.87) * |
| All-cause death | |
| Excluded trial | Median risk ratio (95% credibility interval) |
| MYTHOS trial, 201216 | 0.53 (0.12-1.28) |
| PROTECT TAVI trial, 201517 | 0.48 (0.10-1.16) |
| REDUCE-AKI trial, 201918 | 0.52 (0.15-1.19) |
| REMEDIAL III trial, 202019 | 0.45 (0.08-1.27) |
| CINEMA trial, 202220 | 0.53 (0.16-1.19) |
| STRENGHT trial, 202221 | 0.32 (0.07-1.06) |
| Cardiogenic shock | |
| Excluded trial | Median risk ratio (95% credibility interval) |
| MYTHOS trial, 201216 | 0.10 (0.00-6.52) |
| PROTECT TAVI trial, 201517 | 0.06 (0.00-1.91) |
| REDUCE-AKI trial, 201918 | 0.06 (0.00-1.91) |
| REMEDIAL III trial, 202019 | 0.06 (0.00-1.91) |
| CINEMA trial, 202220 | 0.13 (0.00-9.31) |
| STRENGHT trial, 202221 | 0.06 (0.00-1.91) |
CA-AKI, contrast-associated acute kidney injury.
None of the outcomes evaluated showed significant publication bias on Egger's regression or visual analysis of the funnel plots ().
DISCUSSIONIn this Bayesian meta-analysis, the RenalGuard system was associated with a reduced risk of CA-AKI and acute pulmonary edema, with no difference in all-cause mortality, cardiogenic shock, or acute renal failure requiring renal replacement therapy compared with standard periprocedural intravenous hydration. These results were replicated in several sensitivity analyses, highlighting a valuable role of the RenalGuard system in reducing the incidence of kidney injury after cardiovascular procedures requiring the administration of large amounts of contrast media. Importantly, this outcome was obtained while limiting and even improving the potential consequences of uncontrolled hydration strategies (ie, volume overload and acute pulmonary edema). By means of the Bayesian approach, we were able to show a 95% probability of the RenalGuard system being superior to the control arm in reducing the risk of CA-AKI.
Hydration and minimization of contrast media are essential strategies in CA-AKI prevention.9–14 Tailoring the hydration regimen has been suggested as an alternative to conventional hydration; however, despite several trials and observational registries that have assessed this approach by using the RenalGuard system, the supporting evidence remains scarce and does not allow for definitive conclusions. In particular, the variability of patients included in clinical trials (eg, due to lack of criteria for the selection of the best candidates for the RenalGuard system) resulted in a low-grade level of evidence. Based on this background, our objective was to summarize the most contemporary evidence from the available randomized trials of the RenalGuard system while overcoming some known limitations of frequentist meta-analyses, including their poor ability to estimate pooled treatment effects if there were low event rates due to the use of continuity correction models27 and their overreliance on P values, which do not represent the actual direct probability of response to the treatment.28 Importantly, compared with the previously published meta-analyses, our study focuses on the direct comparison of the RenalGuard system with standard hydration strategies, and adds incremental information by inclusion of the most recent evidence on the topic and avoidance of the variability (ie, “noise”) introduced by indirect comparisons.29 Moreover, the use of random-effects rather than fixed-effects models allowed us to limit the influence of between-trial variability and biological differences on the overall results. We also included a trial sequential analysis, which was not available in other meta-analyses, suggesting that the information size gathered so far in the available randomized trials is sufficient to claim this result to be conclusive.
Although the precise mode of action of the RenalGuard system is not well understood, the high urine output achieved by means of properly matched intravenous hydration in our meta-analysis translated into a significant 46% decrease in the relative risk of CA-AKI. This result is consistent with those of previous studies23,24 and is also in line with several histopathological findings indicating medullary hypoxia as a cause of renal damage through tubular cell injury.30,31 Assuming that volume depletion as a risk factor for renal hypoxia, it can be speculated that achieving a good balance between renal perfusion and proper diuresis has a direct protective effect on the tubular cells, minimizing the risk of injury.32
All our prespecified sensitivity analyses based on varying definitions, timing of assessment (ie, increase of plasma creatinine levels within 48hours from the procedure) and/or diagnostic cutoffs (ie, increase in plasma creatinine levels> 0.5mg/dL) favored, at least directionally, the RenalGuard system. Interestingly, the treatment effect was even higher after exclusion of trials of transcatheter aortic valve implantation, which might be attributed to positive hemodynamic effects after the procedure (ie, due to afterload reduction resulting in improved inotropic cardiac performance) that oppose medullary hypoxia and tubular cell damage, thus downsizing the relative impact of the RenalGuard system.33
Although, from a cost-effectiveness standpoint, the reduction in CA-AKI appears to be attractive,34 it should be acknowledged that the RenalGuard system could represents a potentially costly intervention. A cruder and cheaper approach has recently been tested, based on the idea that an aggressive hydration protocol guided by invasive left ventricle pressure measurements could be beneficial in preventing CA-AKI. However, when compared with standard hydration protocols, the results were controversial.35,36 Moreover, to date, no studies have directly compared RenalGuard with this strategy, and some issues remain unsolved. In particular, because of the requirement for invasive left ventricular pressure measurements to define the fluid rate, the preventive measure can be started only shortly before contrast media administration, neutralizing the potential benefits of more extensive preprocedural hydration. Moreover, as reported in the POSEIDON trial,37 this approach translated into greater fluid administration compared with the standard strategy. These issues, together with the lack of diuresis-matched tracking and with postprocedural pressure measurements, raised some concerns over the application and future of hydration guided by invasive left ventricular pressure measurements as a CA-AKI prevention measure.
Importantly, the benefit of the RenalGuard system did not come at the expense of safety. No difference was observed in the risk of all-cause mortality or cardiogenic shock, and there was even a 65% decrease in the relative risk of acute pulmonary edema. The strong protective association between the RenalGuard system and acute pulmonary edema is an unexpected novel finding with notable implications.23 Indeed, acute pulmonary edema inevitably impacts on patient management and prognosis, particularly when its occurrence is incorrectly attributed to acute pump failure rather than uncontrolled fluid overload, which may lead to useless and even harmful downstream investigations. The beneficial effect of acute pulmonary edema reduction in the investigational group may depend on the use of furosemide, an effect mediated by the blockage of tubular sodium reabsorption in the loop of Henle, which decreases the tubular workload. This effect could lead clinicians to consider furosemide administration alone as a way to prevent acute pulmonary edema. Nevertheless, using furosemide with no adequate matching hydration decreases the effective circulating volume and prostaglandin mediated vasodilation, potentially resulting in dehydration and increasing the risk of tubular damage,38 thus reinforcing the evidence and suggesting a valuable synergic effect of real-time isotonic intravenous hydration matched with high urine output provided by RenalGuard use.
The trial sequential analysis indicated that the information size gathered so far in the available randomized trials is sufficient to claim this result as conclusive. In contrast, we found no differences with the RenalGuard system in reducing renal failure requiring renal replacement therapy. This results is at odds with those of previous meta-analyses showing a lower need for renal replacement therapy, possibly as a result of including heterogenous treatment strategies in the control arm with a low number of events.22,23,39
When discussing the external validity of our results and their real applicability in clinical practice, it is important to point out that all the studies incorporated in our analysis included patients with chronic kidney disease or considered at high-risk for the condition. Therefore, our results demonstrated consistent beneficial effects of RenalGuard use in patients with a high-risk profile. This reinforces the importance of accurate risk stratification and clinical selection to achieve the best result and reduce medical costs.
LimitationsThis meta-analysis has some limitations. The lack of patient-level data allowed us to perform only a study-level meta-analysis, which can be influenced by some residual between-trial variability despite the use of a Bayesian approach. To reduce the influence of such differences, we used random effects models and numerous sensitivity analyses. Some sensitivity analyses lost statistical significance, as a consequence of the relatively small amount of evidence available, but the results remained directionally similar, and the treatment effects were not unduly changed.
CONCLUSIONSIn patients undergoing percutaneous cardiovascular procedures, the use of the RenalGuard system was associated with a reduced incidence of CA-AKI and acute pulmonary edema. These positive effects did not come at the expense of increased mortality or cardiogenic shock.
- –
The evidence on the RenalGuard system as a CA-AKI preventive strategy for patients undergoing cardiovascular procedures requiring iodinated contrast medium administration is sparse.
- –
The available meta-analyses pooled several preventive strategies and used frequentist approaches, which have limitations in drawing definite conclusions.
- –
Using a Bayesian arm-level random-effect model, we concluded that the RenalGuard system is associated with a significant relative reduction of CA-AKI compared with standard hydration, with a degree of evidence that is likely to be conclusive based on trial sequential analysis.
- –
The RenalGuard was found to significantly reduce the risk of acute pulmonary edema and showed no significant safety concerns.
None.
AUTHORS’ CONTRIBUTIONSG. Occhipinti conceived the study and drafted the manuscript; C. Laudani performed the statistical analysis; M. Spagnolo participated in the design of the study; A. Greco participated in the design of the study and reviewed the manuscript; D. Capodanno reviewed the manuscript for intellectual content, and supervised and validated the study. All authors read and approved the final manuscript.
CONFLICTS OF INTERESTNone.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.02.001