The optimal treatment of patients with acute heart failure (AHF) and cardiorenal syndrome type 1 (CRS-1) is far from being well-defined. Arterial hypoperfusion in concert with venous congestion plays a crucial role in the pathophysiology of CRS-I. Plasma carbohydrate antigen 125 (CA125) has emerged as a surrogate of fluid overload in AHF. The aim of this study was to evaluate the clinical usefulness of CA125 for tailoring the intensity of diuretic therapy in patients with CRS-1.
MethodsMulticenter, open-label, parallel clinical trial, in which patients with AHF and serum creatinine ≥ 1.4mg/dL on admission will be randomized to: a) standard diuretic strategy: titration-based on conventional clinical and biochemical evaluation, or b) diuretic strategy based on CA125: high dose if CA125 > 35 U/mL, and low doses otherwise. The main endpoint will be renal function changes at 24 and 72hours after therapy initiation. Secondary endpoints will include: a) clinical and biochemical changes at 24 and 72hours, and b) renal function changes and major clinical events at 30 days.
ResultsThe results of this study will add important knowledge on the usefulness of CA125 for guiding diuretic treatment in CRS-1. In addition, it will pave the way toward a better knowledge of the pathophysiology of this challenging situation.
ConclusionsWe hypothesize that higher levels of CA125 will identify a patient population with CRS-1 who could benefit from the use of a more intense diuretic strategy. Conversely, low levels of this glycoprotein could select those patients who would be harmed by high diuretic doses.
Keywords
Renal failure commonly occurs in heart failure (HF), where it contributes adversely to major clinical outcomes.1–3 Indeed, a high proportion of patients admitted for acute heart failure (AHF) shows renal dysfunction on admission or develops worsening of renal function (WRF) during hospitalization.3 The coexistence of these 2 conditions has translated in longer hospital stays and worse short- and long-term prognosis.4,5 In the setting of AHF, the pathophysiology of renal dysfunction is complex and not fully understood.3 While traditionally attributed to the reduction in cardiac output and the consequent renal hypoperfusion, recent data have highlighted the role of renal congestion as an alternative pivotal mechanism.6–9 Building on these findings, we believe that it is crucial to identify those patients who might benefit from tight control of volume overload. Because the intensity of diuretic therapy usually relies on subjective evaluation and not on evidence-based guidance, it has become an unmet need the search for a tool that not only reflects the severity of fluid overload but is also useful for tailoring the intensity of diuretic therapy. Based on data showing that plasma levels of carbohydrate antigen 125 (CA125) are strongly correlated with clinical, hemodynamic, and echocardiographic surrogates of fluid overload,10–15 together with recent findings of a clinical trial endorsing the role of CA125 for guiding therapy in patients with a recent episode of AHF,16 we speculate that this biomarker may serve as a potential candidate for guiding diuretic therapy in patients with cardiorenal syndrome type 1. Under the proposed hypothesis, high diuretic doses would be especially beneficial in terms of renal function and clinical status in patients with increased CA125. In contrast, low CA25 would identify those patients who should undergo a more conservative diuretic strategy.
METHODSStudy DesignThis is an investigator-initiated, multicenter, open-label, parallel clinical trial, in which patients with AHF will be randomized to 1 of 2 following strategies: a) standard diuretic strategy with a titration scheme based on standard evaluation, or b) diuretic strategy based on the plasma levels of CA125. Due to the inherent characteristics of this design, complete blinding of the physician is not feasible; patients, however, will be blinded to the treatment groups. The minimum duration of patient participation is 30 days. The study is being conducted in 9 centers in Spain and in accordance with the principles of Good Clinical Practice, as expressed by the Declaration of Helsinki. All patients provided signed informed consent and the protocol was approved by the research ethics committee of participating centers and Agencia Española de Medicamentos y Productos Sanitarios. This study is registered in ClinicalTrials.gov (NCT02643147).
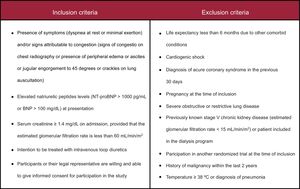
Study PopulationThe study population consisted of patients with AHF and renal dysfunction requiring hospital admission or intravenous diuretic administration in an ambulatory setting. These patients will be randomized during the first 24hours after presentation. Inclusion and exclusion criteria are presented in the Figure.
RandomizationAfter providing informed consent, patients were randomly assigned, with a remote, web-based computer-generated block randomization procedure in a n allocation 1:1 ratio, to either the CA125 strategy or standard diuretic strategy.
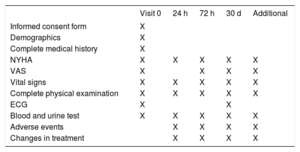
Study ProceduresScreening and Eligibility Assessment (Visit 0)Patient screening including laboratory tests and procedures are conducted at the time of presentation along with verification of inclusion and exclusion criteria.
The following procedures or data collection will be performed after fulfillment of the inclusion criteria: a) signed and dated informed consent form; b) demographic data; c) complete medical history including current treatment and prior medications received within the last 30 days; d) last functional class evaluation (New York Heart Association) before decompensation; e) assessment of dyspnea by using the visual analog scale; f) vital signs and complete physical examination; g) electrocardiogram; h) urinalysis, and i) blood test, including hematology, natriuretic peptides, troponin, CA125 and renal function parameters (serum creatinine [Cr], urea nitrogen, estimated glomerular filtration rate, and cystatin C). Estimated glomerular filtration rate will be estimated using the Modification of Diet in Renal Disease formula.
Follow-up VisitsScheduled follow-up visits will be performed at 24hours, 72hours, and 30 days after randomization (final visit). At these visits, data on vital signs, complete physical examination, functional class (New York Heart Association) and visual analog scale evaluation will be registered. Laboratory tests will include urinalysis, serum Cr, urea, blood urea nitrogen, estimated glomerular filtration rate, cystatin C, and B-type natriuretic peptide/N-terminal pro-B-type natriuretic peptide. During the follow-up, patients will be censored if they die. Discontinuation will occur if they withdraw their informed consent.
Additional VisitsVisits outside this preplanned schedule (optional visits) will be permitted at the discretion of the physician in charge of the patient. The main reason for each optional visit, and any laboratory or procedure performed must be recorded on the chart and clinical monitor must be informed.
Summary of study procedures are detailed in Table 1.
Follow-up Design
| Visit 0 | 24 h | 72 h | 30 d | Additional | |
|---|---|---|---|---|---|
| Informed consent form | X | ||||
| Demographics | X | ||||
| Complete medical history | X | ||||
| NYHA | X | X | X | X | X |
| VAS | X | X | X | X | |
| Vital signs | X | X | X | X | X |
| Complete physical examination | X | X | X | X | X |
| ECG | X | X | |||
| Blood and urine test | X | X | X | X | X |
| Adverse events | X | X | X | X | |
| Changes in treatment | X | X | X | X |
ECG, electrocardiogram; NYHA, New York Heart Association functional class; VAS, visual analog scale for dyspnea.
During the study period, information on concomitant medications and clinical adverse events (death from all causes or new worsening of AHF) will be recorded.
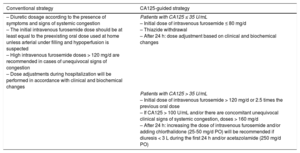
Trial InterventionEligible patients will be randomized to receive a conventional diuretic strategy (driven by clinical assessment) or a diuretic strategy guided by plasma CA125 levels. All diuretics and concomitant medications used in this trial are approved for HF treatment and are part of standard of care in this entity.17,18 Detailed treatment algorithms are presented in Table 2.
Diuretic Treatment Strategies
| Conventional strategy | CA125-guided strategy |
|---|---|
| – Diuretic dosage according to the presence of symptoms and signs of systemic congestion – The initial intravenous furosemide dose should be at least equal to the preexisting oral dose used at home unless arterial under filling and hypoperfusion is suspected – High intravenous furosemide doses > 120 mg/d are recommended in cases of unequivocal signs of congestion – Dose adjustments during hospitalization will be performed in accordance with clinical and biochemical changes | Patients with CA125 ≤ 35 U/mL – Initial dose of intravenous furosemide ≤ 80 mg/d – Thiazide withdrawal – After 24 h: dose adjustment based on clinical and biochemical changes |
| Patients with CA125 > 35 U/mL – Initial dose of intravenous furosemide > 120 mg/d or 2.5 times the previous oral dose – If CA125 > 100 U/mL and/or there are concomitant unequivocal clinical signs of systemic congestion, doses > 160 mg/d – After 24 h: increasing the dose of intravenous furosemide and/or adding chlorthalidone (25-50 mg/d PO) will be recommended if diuresis < 3 L during the first 24 h and/or acetazolamide (250 mg/d PO) |
CA125, carbohydrate antigen 125; PO, per os.
The diuretic dosage will be selected according to the presence of symptoms and signs of systemic congestion and according to current recommendations.17,18 The initial intravenous furosemide dose should be at least equal to the preexisting oral dose used at home unless arterial under filling and hypoperfusion is suspected. Keeping the scheduled starting dose for at least the first 24hours of admission will be advisable. The diuretic regimen will be subject to revision based on patient response and laboratory criteria. Administration of additional diuretics and other drugs (nitrates, antiarrhythmics, inotropes, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor neprilysin inhibitors, beta-blockers, and digoxin) are left to the discretion of the attending physician. Initiation of renal replacement therapy should be considered in patients with refractory congestive HF at the judgment of the attending physician and/or severe hyperkalemia (> 6.5 mmol/L) and/or severe acidemia (pH < 7.2).
Carbohydrate Antigen 125-guided StrategyIn this group, the diuretic regimen will be titrated according to CA125 serum levels:
- –
Patients with CA125 ≤ 35 U/mL. An initial dose of intravenous furosemide ≤ 80mg/d will be recommended–this is regardless of any previous dose the patient was receiving. Maintaining this dose for the first 24hours will be advised. Discontinuation of aldosterone receptor blockers, thiazides, or other diuretics will also be recommended. After the first 24hours, any further decision regarding initial dose modification or route of administration of loop diuretics will be at the discretion of the attending physician based on patient's clinical and biochemical response.
- –
Patients with CA125 > 35 U/mL. An initial dose of intravenous furosemide > 120mg/d (or 2.5 times the oral dose the patient was receiving) will be set as the standard for this group. Doses > 160mg/d will be recommended for those with CA125 > 100 U/mL and clinical signs of severe systemic congestion. After 24hours, the physician in charge of patient care will be allowed to make changes about dosage, route of administration, or, if necessary, stop diuretic treatment. As a general rule, if a diuresis achieved during the first 24hours is ≤ 3 L, an increase in the dose of intravenous furosemide and/or adding chlorthalidone 25-50mg/d will be recommended unless there is important impairment of renal function (increase > 0.5mg/dL in Cr relative to admission). The preferred method of intravenous furosemide administration for doses > 250mg/d of furosemide is by continuous infusion; otherwise, bolus will be used.
In all patients, oral loop diuretics must not be administered together with intravenous loop diuretic administration. The recommendations on the use of other drugs (nitrates, antiarrhythmics, inotropes, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, angiotensin receptor neprilysin inhibitors, beta-blockers and digoxin) and renal replacement therapy are the same as those suggested for the conventional-arm.
EndpointsPrimary EndpointThe main endpoint is to assess changes in renal function at 24 and 72hours after enrollment. To this end, the following markers of renal function will be measured: serum Cr, urea, urea to Cr ratio, estimated glomerular filtration rate, and cystatin C. Changes in renal function parameters will be evaluated as continuous (absolute and relative) and categorical. Improved renal function and WRF and will be defined by a Cr increase and a decrease in Cr of at least 0.3mg/dL, respectively.
Secondary EndpointsThe following secondary endpoints will also be compared between the 2 strategies:
- –
Resolution of the breathlessness (changes in New York Heart Association functional class), signs of systemic congestion, and patient global assessment (by visual analog scale) at 24 and 72hours after enrollment.
- –
Changes in plasma levels of natriuretic peptides (B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide) at 72hours after enrollment.
- –
Changes in plasma levels of troponin at 72hours after enrollment.
- –
Changes in urinalysis at 24 and 72hours (Cr, sodium and potassium).
- –
Time required to change intravenous diuretics to oral administration.
- –
Parameters of renal function (Cr, urea, urea to Cr ratio, glomerular filtration rate and cystatin C) at 30 days.
- –
Adverse clinical events: combined adverse event of death and/or rehospitalization for AHF at 30 days.
A strict policy will be implemented regarding a close adverse event surveillance to ensure early detection and appropriate management of renal deterioration, and/or severe electrolyte and pH disturbances. Therefore, the following existing conditions that occur following enrollment, should be monitored, treated and recorded: a) increase in serum Cr to ≥ 300% or serum Cr of ≥ 4mg/dL (≥ 354 mmol/L) with an acute increase of at least 0.5mg/L (44 mmol/L) compared to prerandomization serum levels; b) hyperkalemia (serum potassium > 6.0 mEq/L): in cases where diuretic treatment modality may be implicated, a special review of diuretic treatment should be performed. In these cases, aldosterone antagonist should be withdrawn, and c) hypokalemia (serum potassium < 3.0 mEq/L): all cases should be reported and supplementation will be considered, in particular in patients being treated with aldosterone antagonists.
Sample Size CalculationA 2-tailed Fisher exact test was used for sample size calculation. The estimated sample size for improvement of renal function was 77 patients in each group (total 154 patients), and was 39 patients per group for WRF (total 78 patients). Assuming a loss of 5% to 10% of patients (consent withdrawn, lost to follow-up at 30 days, and early deaths) we increased the sample size by 10%, leaving the final sample at 170 patients. The effect size of interest (or significant minimum size) and the probability of the outcomes (improvement of renal function and WFR) in group 1 were obtained from a pilot study conducted in our department.19 More detailed information about the sample size estimation is provided in the supplementary material.
Statistical AnalysisAll statistical comparisons will be made under an intention-to-treat principle. Data will be presented as mean ± standard deviation for normally-distributed variables, and as median with interquartile range otherwise. Discrete data are expressed as frequency and percentages. Continuous and normally-distributed variables will be tested among the 2 strategies by the unpaired Student t test or by the Wilcoxon rank-sum test. The Fisher exact test will be used to compare 2-level discrete variables; multicategorical variables will be compared with chi-square tests.
Primary EndpointThe null hypotheses stated that there are no differences in the delta change of Cr among strategies–absolute changes in Cr between admission and 72hours ranging from -0.29 to 0.29mg/dL. Absolute change in Cr will be compared between the 2 strategies at 24 and 72hours after enrollment with ANCOVA (analysis of covariance), using the baseline value of Cr as covariate. In the event that a random-driven imbalance occurs in some of baseline characteristics, such variable(s) will also be used as covariates in the ANCOVA model. A Bonferroni correction will be applied by setting the value of alpha at 0.025 to account for multiple comparisons between the 3 subgroups: control, CA125 ≤ 35 U/mL, CA125 > 35 U/mL.
Secondary EndpointDifference among strategies for the combined 30-day death and/or rehospitalization for AHF endpoint will be graphically depicted by the Kaplan-Meier method and tested by the log-rank test. Univariate hazard ratios will be estimated with Cox proportional hazard regression; the main censoring criteria will be the end of the trial (administrative censoring at 30 days). Multivariable Cox regression analysis will be used only in the event that important prognostic factors or patient characteristics at baseline show a significant imbalance between the 2 randomized groups. All analyses will account for a potential clustering effect within centers. Missing values will be imputed multiple imputation techniques, under the assumption of MCAR (missing completely at random) or MAR (missing at random).
A 2-sided P-value of < .05 will be considered statistically significant for all analyses. All analyses will be performed using Stata 14.2 (StataCorp. 2015. Stata Statistical Software: Release 14.2 College Station, Texas: StataCorp LP).
Status of the TrialPatient enrollment started in April 2015. On November 2, 2016, 142 patients have been enrolled in the study (83.5% of the target).
DISCUSSIONRenal dysfunction is a pervasive companion in patients with HF with higher figures reported during AHF decompensations.1–3 As a consequence, these patients have longer hospital stays and generally worse prognosis in the short- and long-term.4,5
An additional complicating factor is that at greater degrees of renal failure, well-evidenced therapies are lacking and current management remains mostly empirical.3,17,18
The pathophysiology of renal dysfunction in patients with AHF is complex, multifactorial, and not fully elucidated.3 Historically, WRF has been attributed to the reduction in cardiac output and consequently to renal hypoperfusion.3 However, recent data have failed to correlate surrogate parameters of low cardiac output with WRF in patients with HF.3,9 Indeed, a recent and elegant study of Hanberg et al.20 found no association between reduced cardiac index–assessed by pulmonary artery catheterization–and degree of renal dysfunction in 575 patients with advanced HF (New York Heart Association functional class III or IV, and mean left ventricular ejection fraction 23 ± 12%). Likewise, there are also recent data highlighting the role of renal congestion (venous, intra-abdominal, and hydrostatic pressure of the renal vein) as an important player in the pathogenesis of WRF complicating AHF.6–9 For example, Mullens et al.,8 found that the elevation of the central venous pressure on admission and the persistence of this elevation after treatment were the most important factors associated with WRF. In a related context, Damman et al.,21 showed, in 30 patients with chronic congestive HF, an increase in markers of renal tubular injury after the withdrawal of diuretics. Similar findings have been corroborated in other studies, emphasizing the role of venous congestion as a potential contributor in the pathophysiology of renal dysfunction, especially in patients with AHF.6–9
Currently, diuretics are the pharmacological therapy of choice for the treatment of congestion in AHF patients.17,18 However, its use is largely empirical and has traditionally been associated with significant deleterious effects, including WRF.3,17,18,22,23 Paradoxically, in some subsets of patients, diuretics have also been shown to be independently associated with improved renal function,3,19,21,23 a finding that, at least indirectly, reinforces the role of venous congestion as the main player in the deterioration of glomerular filtration rate in AHF. It seems that the effects of diuretics on renal function are determined by a delicate balance between renal perfusion pressure and renal venous congestion.3 Unfortunately, no consensus exists about the best way to guide diuretic therapy in these patients.17,18 Perhaps the absence of definitive evidence from randomized clinical trials together with the fact that no reliable method exists for quantification of systemic congestion explain the lack of a clinical tool for diuretic-guiding therapy.24,25 Indeed, common clinical parameters used for diagnosis and prognostic stratification have shown poor profitability for quantifying the degree and distribution of fluid overload.24 In addition, it is worth mentioning that natriuretic peptides levels have shown no correlation with the degree of congestion.24,26 Therefore, in the AHF setting, there is an unmet need for a clinical/biological tool that reflects the severity of the fluid overload while being sensitive enough to detect variations induced by diuretic therapy.
In this line of thought, plasma CA125 levels have emerged as a potential candidate for guiding depletive therapy.15 Carbohydrate antigen 125 is a glycoprotein synthesized by epithelial serous cells that has been widely used for monitoring ovarian cancer therapy. However, high plasma levels have also been reported in other diseases such as HF, nephrotic syndrome, liver cirrhosis, or pelvic inflammatory disease, among others.15 In the HF setting, several studies have shown that CA125 strongly correlated with clinical, echocardiographic, and hemodynamic parameters indicative of severity of systemic congestion and/or organ water redistribution.10–15 In addition, elevated levels of this glycoprotein are present in most patients hospitalized for AHF and are associated with an increased risk of death and readmission for AHF.10–15 Moreover, this biomarker has proved to be sensitive enough to follow the patient's clinical course; as such, it has been tested as a potential tool for monitoring and guiding depletive treatment in HF.27–31 In fact, in a recent randomized study performed in 380 patients with a recent episode of AHF, a CA125-guided therapy, driven mainly on modifications of frequency of monitoring and the intensity of diuretic therapy, was associated with a significant reduction in 1-year risk of subsequent AHF-readmissions compared with standard of care.16 In this trial, the authors suggested an intensification of the frequency of monitoring and up-titration of diuretics when CA125 was elevated (CA125 > 35 U/mL) while following a more relaxed schedule of ambulatory visits and down-titration of diuretic therapy if CA125 was ≤ 35 U/mL.16 Along the same line, but in the AHF setting, we found that, in 526 patients hospitalized for AHF, early changes in renal function following intravenous administration of furosemide were largely dependent on renal function and plasma levels of CA125 on admission. In fact, increasing doses of furosemide were associated with an increase in serum Cr at 48 to 72hours in patients with Cr ≥ 1.4mg/dL on admission and CA125 < 35 U/mL.19 In contrast, increasing doses of furosemide were associated with a significant decrease of Cr in subjects with Cr ≥ 1.4mg/dL on admission and CA125 > 35 U/mL. Similar findings for blood urea nitrogen values and estimated glomerular filtration rate were observed.19 Moreover, in 1389 patients admitted for AHF, our group observed that the mortality risk associated with the dose of loop diuretics at discharge was intense and differentially associated with plasma levels of blood urea nitrogen and CA125.32 Thus, high doses of loop diuretics were associated with an increased risk of death during follow-up, especially in patients with normal CA125 values and high levels of blood urea nitrogen, while in those who had elevated levels of blood urea nitrogen and CA125, high doses of loop diuretics were associated with a 27% decrease in the risk of death.32
Other characteristics such as its wide availability, low cost, standarized measurement, prolonged half-life, and the fact that CA125 levels are not subtantiallly influenced by age and renal dysfunction are important advantages that deserve to be highlighted.15
In summary, a substantial body of evidence suggests the usefulness of this biomarker for monitoring and guiding diuretic therapy in patients with cardiorenal syndrome type 1. A randomized clinical trial is a necessary step forward to evaluate this hypothesis.
CONCLUSIONSThere is enough evidence to support the notion that, in patients hospitalized for AHF, early changes in renal function induced by the administration of diuretics are influenced by the degree of venous congestion. Under these premises, we postulated that the use of high doses of diuretics would be especially beneficial–in terms of improvement of renal function–in patients with WRF and increased plasma levels of CA125 on admission. In contrast, high doses of diuretics would be associated with greater renal deterioration in patients with WRF and serum levels of CA125 within normal limits on admission.
FUNDINGProject PI13/01519 in collaboration with “Plataforma de Unidades de Investigación Clínica y Ensayos Clínicos” (SCReN [Spanish Clinical Research Network]) (PT13/0002/0031). Cofinanciated by ERDF (European Regional Development Fund). Unrestricted grant: “Proyectos de Investigación de Insuficiencia Cardiaca de la Sección de Insuficiencia Cardiaca 2015” from “Sección de Insuficiencia Cardíaca de la Sociedad Española de Cardiología”. Unrestricted grant: “Beca Mutual Médica 2014”. Instituto de Salud Carlos III and co-funded by ERDF [grant number PIE15/00013]. Red de Investigación Cardiovascular; Programa 7 (RD12/0042/0010)
CONFLICTS OF INTERESTNone declared.
- –
Renal failure commonly occurs in AHF, where it contributes adversely to major clinical outcomes.
- –
Recent data have highlighted the role of renal congestion in the pathophysiology of renal dysfunction in patients with AHF.
- –
There is a lack of well-evidenced therapies in this setting and the intensity of diuretic therapy usually relies on subjective evaluation rather than on evidence-based guidance.
- –
Plasma levels of CA125 are strongly correlated with clinical, hemodynamic, and echocardiographic surrogates of fluid overload.
- –
We speculate that CA125 may serve as a potential candidate for guiding diuretic therapy in patients with cardiorenal syndrome type 1.
- –
High doses of diuretics would be especially beneficial, in terms of improvement of renal function, in patients with WRF and increased plasma levels of CA125 on admission.
- –
In contrast, high doses of diuretics would be associated with greater renal deterioration in those with WRF and serum levels of CA125 within normal limits on admission.
Amparo Villaescusa, Anna Mollar, Antonio Gabarrón and Paula Lizandra.