Keywords
Intravascular stent implantation for the treatment of vascular stenoses has advanced considerably in recent years, although use of this technique for congenital heart disease in newborns is extremely limited.
In cases of pulmonary atresia with intact ventricular septum (PA/IVS) and in critical pulmonary stenosis (CPS), pulmonary circulation after birth depends on the ductus arteriosus, which requires intravenous use of prostaglandin E1 (PGE1) to maintain patency. Pulmonary balloon valvuloplasty is performed for both these conditions, although in PA/IVS the valve must be perforated during cardiac catheterization, either mechanically1,2 or with radiofrequency3,4 or laser5 techniques. Once the obstruction of the right ventricle is released, biventricular physiology is not always achieved immediately, making it necessary to continue PGE1 therapy and in some cases to create a Blalock-Taussig shunt (BTS). This last option has been associated with complications, such as phrenic or vagal nerve paralysis, chylothorax, distorted pulmonary artery growth, pulmonary branch stenosis, and surgical adhesions. Hence, a nonsurgical option for BTS6 is necessary. Stent implantation to maintain ductus arteriosus patency7,8 has been used in diseases with duct-dependent systemic blood flow or pulmonary blood flow, with much better results in the latter indication. In fact, the best results are obtained in diseases where the ductus arteriosus is short and less tortuous, such as PA/IVS and CPS.6,9 We report on our experience with stent implantation to maintain the patency of the ductus arteriosus and thereby avoid palliative surgery.
PATIENTS AND METHODS
Patients
Three patients (Table 1) were studied by echocardiography prior to catheterization and considered to have a tripartite right ventricle. In patients 1 and 2, right ventriculography was performed during catheterization, and the coronary-ventricular fistulas were considered non-significant.
Technique
Informed consent was obtained in all cases before the procedure. Stent implantation was performed by an anterograde approach. A 0.014-inch guidewire was advanced through the femoral vein to the femoral artery, across the right atrium, through the pulmonary artery, ductus arteriosus and descending aorta, and ultimately exteriorized through the femoral artery to establish a venoarterial loop. All stents were premounted on a balloon (Stent BX Sonic®, 4x18 mm, Cordis/Johnson & Johnson) and advanced coaxially over a 0.014-inch guidewire. Stent implantation was done under radioscopic control, and a lateral aortogram was performed to verify the stent position. PGE1 perfusion was discontinued in all cases before stent implantation, in order to visualize on angiography the area of maximum ductal constriction, which was used as a reference to adjust to optimal stent position during expansion. During the catheterization, a control echocardiogram was performed to ensure that the stent was correctly positioned in the duct. All children received intravenous cefazolin during the procedure. In addition all received aspirin 5 mg/kg/day and dipyridamole 3 mg/kg/day for 3 months.
RESULTS
Patient 1
A newborn with PA/IVS underwent a procedure to open the pulmonary valve, but continued to depend on PGE1 to maintain a SatO2 of 85%-90%. Since it was impossible to discontinue therapy, a decision was made to implant a ductal stent 17 days later. PGE1 perfusion was discontinued during implantation of the ductal stent; SatO2 at the end of the procedure was 92%.
Patient 2
A newborn with PA/IVS underwent radiofrequency ablation and balloon valvuloplasty. Echocardiography performed in the interventional cardiology suite showed slow anterograde flow through the pulmonary valve and ductal constriction despite PGE1. A decision was made for immediate placement of a ductal stent and discontinuation of PGE1 perfusion. SatO2 at the time of discharge was 88%.
Patient 3
An infant with CPS required PGE1 perfusion after valvuloplasty to maintain SatO2>90%; 2 days later, he presented clinical symptoms of ductal closure (Figure, A) and, despite high PGE1 doses, SatO2 persisted at 68%-70%. A ductal stent was implanted urgently and SatO2 at the time of discharge was 90%-93%.
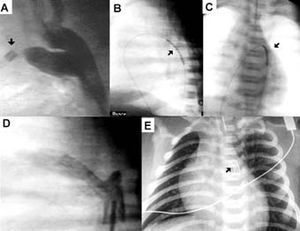
Figure. Patient with critical pulmonary stenosis. A) aortography, see other catheter (arrow) in the pulmonary artery showing ductal constriction. B) and C) balloon-mounted stent (arrow) prior to inflation; side and anteroposterior view. D) aortography after stent placement. E) chest x-ray in which the stent can be observed (arrow).
Follow-up
None of the patients required resumption of PGE1 perfusion or emergency BTS during the acute period. The follow-up data are shown in Table 2; in all patients, the ductus arteriosus remained open and there were no clinical symptoms of pulmonary overflow or any need for diuretics. Pulmonary pressure was normal in all patients, and right ventricular pressure was also normal except in the patient with CPS, who had residual pulmonary stenosis on echocardiography.
DISCUSSION
Two representative series10,11 of patients with PA/IVS who underwent valve perforation by radiofrequency reported that 52% (14/27 and 17/33) of the patients required a BTS between 2 and 24 days after therapeutic catheterization. In CPS it has been observed that 7% of the patients needed a BTS after pulmonary valvuloplasty.12
At the present time, the factors predicting which patients will need a BTS after releasing the obstruction of the right ventricular outflow tract are not accurately known.13 Identifying these factors would allow us to stratify the patients and create a BTS without waiting too long or doing it as elective surgery. The effectiveness of stenting in certain lesions with duct-dependent pulmonary circulation was questioned by Gibbs et al,8 although all patients in this series had a diagnosis of pulmonary atresia with ventricular septal defect (VSD), a disease in which the ductus arteriosus is generally long and tortuous.6 In contrast, Schneider et al9 reported that stent implantation was successfully performed in their series of eight patients with a history of PA/IVS or CPS, with no mortality or need to resume prostaglandin therapy. This results from the fact that the ductus arteriosus in CPS and PA/IVS is short and straight.
The ductal stent has been observed to be completely endothelialized at 30 days.8 Stent patency decreases over time; in 4 of 6 patients it was completely occluded between 4.5 and 17 months (mean, 10 months), a desirable outcome in these patients since it indicates that the right ventricle has already adapted to biventricular physiology.9
Our brief experience reflects three different situations. In patient 1, it was necessary to implant the stent electively 17 days later because PGE1 perfusion could not be discontinued. Patient 2 required immediate stent implantation because the echocardiogram in the catheterization room showed that, although the pulmonary valve had opened completely, there was almost no pulmonary anterograde flow and ductal restriction existed despite PGE1 infusion. In the third patient, sudden closure of the ductus required urgent stent implantation 2 days later.
COMMENTS
Although our initial experience is limited, we can suggest the advantages offered by stent placement in this group of patients: a) avoidance of surgical creation of BTS; b) relatively simple implantation, further facilitated when the femoral vessels are still canalized after the outflow tract is completely opened; c) temporary implantation: both ductus arteriosus stenting and the BTS are temporary solutions, since we hope to achieve biventricular viability in these patients (in patients in whom this evolution is not possible, the next step after several months would be a Glenn shunt as an initial step to single-ventricle repair or one and a half ventricular repair; d) possibility to redilate the ductus arteriosus stent in cases where it becomes restrictive; e) easier closure of the ductus arteriosus when it is no longer necessary, using percutaneous techniques; f) avoidance of known distortion of the pulmonary arteries after BTS; and g) avoidance of prolonged PGE1 therapy.
One problem we found in this article that should be studied in the future is how to identify the group of patients (about 50% and 7% in PA/IVS and CPS, respectively) who will need prolonged support with PGE1, BTS, or a ductal stent after the right ventricle is open.
Correspondence: Dr. C. Mortera Pérez.
Servei de Cardiologia. Hospital Sant Joan de Déu.
Passeig de Sant Joan de Déu, 2. 08950 Barcelona. España.
E-mail: cmortera@hsjdbcn.org
Received June 23, 2004.
Accepted for publication October 5, 2004.