Keywords
INTRODUCTION
Atrioventricular conduction problems are a common complication of acute myocardial infarction (AMI) involving the inferior wall. These complications are associated with increased mortality,1-7 and are therefore a sign of poorer prognosis. In the pre-thrombolytic era, the incidence of advanced atrioventricular block (second or third degree) in inferior wall AMI was some 19%.5 Although this has been reduced to 11%-12%1,3,8,9 since the introduction of fibrinolytic treatment, it remains one of the most important complications. The efficacy of fibrinolytic treatment is greater the earlier it is provided.10-13 In inferior wall AMI with complete atrioventricular block (CAVB), the dilemma of whether to implant a provisional pacemaker--a course of action that might delay the start of fibrinolytic treatment--is often faced. Implantation also involves a risk of hemorrhage at the puncture site.
The literature contains information on the incidence and development of CAVB in inferior wall AMI in the pre-thrombolytic era, but the duration of CAVB in fibrinolysis-treated patients has not been reported.1,6,8 The aim of the present work was to analyze the development and characteristics of CAVB in patients admitted with inferior wall AMI who were candidates for fibrinolytic treatment, and to compare the results with those of patients who received no such treatment.
PATIENTS AND METHODS
Between 1 January 1992 and 31 January 2002, 1134 consecutive patients were admitted to our heart unit with inferior wall AMI. Of these, 552 were brought directly to our facility; the remainder were referred from other centers. Four hundred and forty nine patients arrived within six hours of onset (within the time window for fibrinolysis), and 282 (64%) received thrombolytic treatment. Of this group, 39 (13.8%) presented with CAVB (group A). Thrombolytic treatment was not administered to the remaining 167 patients (i.e., of these 449; 36%) since electrical criteria were not fulfilled, because such treatment was clearly contraindicated, or for reasons of age or delay in arriving at a definitive diagnosis of AMI, etc. Of these 167 who did not undergo fibrinolysis, 13 (8%) presented with CAVB (group B, control). The evolution of CAVB in the two treatment groups was compared.
The criteria for administering fibrinolytic treatment were: chest pain lasting longer than 30 min, an ST segment elevation of ≥1 mm from 2 consecutive leads, <6 h having elapsed since the onset of symptoms, and the complete absence of contraindications. A final diagnosis of AMI was established according to classic criteria given the presence of at least 2 of the following 3 conditions: creatine kinase (CK) levels more than double the reference value, ischemic chest pain lasting more than 30 min, or the appearance of pathological Q waves lasting >0.04 s in the electrocardiogram (ECG). Involvement of the right ventricle was diagnosed by electrical (ST segment elevation in V3R or V4R), and clinical and echocardiographic (dilation and alteration of contractility) criteria.
The following variables were recorded in both group A and B: the characteristics of the patients, the chronology and duration of CAVB, the time elapsed between the onset of pain and arrival at the emergency department, the time of starting fibrinolytic treatment, specific treatment for CAVB, enzyme peaks, and in-hospital mortality.
Statistical Analysis
SPSS® software v.11.0 for Windows was used for all statistical analyses. The distribution of the variables was analyzed using the Kolmogorov-Smirnov test. The Student t test was used to analyze continuous quantitative variables that showed a normal distribution (age, CK and CK-MB peaks, time to presentation of CAVB, and the time elapsed until the start of fibrinolytic treatment); results are expressed as means±standard deviation. The Mann-Whitney test was used to compare quantitative variables whose distributions were not normal (such as the duration of CAVB); values are expressed as medians and interquartile ranges. Dichotomous variables (sex, coronary risk factors, angina, previous AMI, etc) were compared using the χ² test. Significance was set at P<.05.
RESULTS
Patient Characteristics
The mean age of the 39 patients of group A was 61±12 years. Of these patients, 35 (89%) were men. The mean age of the control group (group B) was 67±12 years; 9 (69%) were men (P=NS). Table 1 shows all the patient characteristics recorded.
Characteristics of the Acute Myocardial Infarction Suffered
In group A, 16% of patients presented with a Killip class III-IV AMI; in group B 54% of the patients presented with an AMI of these characteristics (P=.02) (Table 2).
The right ventricle was involved in 17 group A patients (41%), although only in 12 (30%) was there a significant clinical repercussion. All patients showed increased enzyme levels. The CK peak was 3.304±2.077 U/L and the CK-MB peak 335±117 ng/mL.
The right ventricle was involved in 5 group B (38%) patients. The CK peak was 2.894±2.161 U/L and the CK-MB peak 261±158 ng/mL. No significant differences were seen between the 2 groups. The determination of troponin levels was not standard practice for most of the study period; these data are therefore not shown.
Fibrinolytic Treatment
The mean time elapsed between the onset of pain and arrival at the emergency department was 80±53 min for group A and 137±73 min (P=.004) for group B. The mean time elapsed between the onset of pain and the start of fibrinolytic treatment in group A was 151±67 min. The thrombolytic agents used were streptokinase (in 73% of cases), tissue plasminogen activator (in 19% of cases) or other agents (8% of cases).
Characteristics and Treatment of Atrioventricular Block
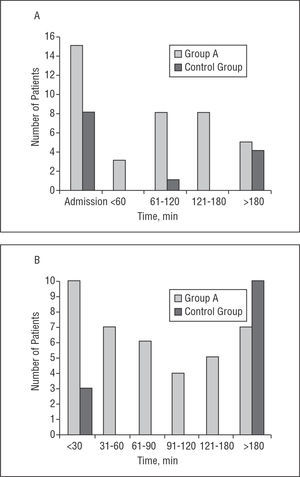
Of the 39 patients in group A, 15 (38%) showed CAVB on arrival, as determined by the initial ECG. In group B, CAVB was noticeable in the initial ECG of 8 patients (62%) (P=NS). Of the 24 remaining patients in group A, the median time to the start of CAVB since the onset of pain was 150 min (range, 45 min to 48 h). In 84% of cases, CAVB appeared in the first 3 h following the onset of pain. In 2 patients, CAVB appeared 48 h after pain onset, without being preceded by further chest pain or re-elevation of the ST segment.
In group A, the median duration of CAVB was 75 min (P25 15 min, P75 120 min) (range, 10 min to 48 h); in group B, CAVB lasted a median of 1440 min (P25, 15 min, P75 5760 min) (range, 15 min to 9 days) (P=.004). When fibrinolysis was begun, the CAVB rapidly reverted to sinus rhythm. The median duration of the block from the time thrombolysis began was 45 min (range, 5 min to 48 h). Omitting the patient in whom CAVB lasted 48 h, the mean duration of blockage after the start of fibrinolysis was 51 min. In 69% of group A patients the duration of CAVB was <2 h, while in 50% of group B patients it lasted more than 24 h, and in 77% more than 4 h (Figure).
Figure 1. A: Time to presentation of CAVB since the onset of pain (minutes). B: duration of CAVB (minutes). CAVB indicates complete atrioventricular block.
Decisions on the pharmacological management of the patients and the need to implant a provisional pacemaker were taken by the attending physician (no guidelines on the treatment of AMI were available from the Sociedad Española de Cardiología until 1999). In group A, 18 patients (46%) received atropine and 3 (16.6%) reverted to sinus rhythm. Six patients (15%) received dopamine, 4 of whom (66%) recovered their sinus rhythm with 1:1 conduction in a short time. Only 2 patients received an intravenous perfusion of isoproterenol for extreme bradycardia (<40 beats/min). Although this did not revert the CAVB, the ventricular escape rate was improved and a provisional pacemaker implanted. Atropine was administered to 23% of group B patients (P=NS) but in only 1 was the CAVB reverted to sinus rhythm. Eight of these patients (61%) were administered dopamine and 2 received isoproterenol (P=NS).
In group A, 17 patients (43%) were intravenously implanted with a provisional electrocatheter whereas in group B 11 patients (84,6%) required this procedure (P=.01). In 12 (30%) of the 39 group A patients, CAVB was present with low blood pressure (systolic blood pressure <90 mm Hg) and severe bradycardia (heart rate <40 beats/min); no response to atropine was observed. All 12 were implanted with a provisional pacemaker. In the remaining 5 patients, the electrocatheter was implanted as prophylaxis against CAVB-associated hemodynamic deterioration. In group B, 10 of the 11 patients who needed transitory stimulation showed clear hemodynamic deterioration (low blood pressure and severe bradycardia). In the remaining patient, prior serious syncope was taken to indicate the need for a pacemaker.
In all group A patients, access was possible via the femoral vein (mostly the right). In four of the 17 above patients (23%), the pacemaker was implanted before fibrinolytic treatment. In group B, access was obtained via the right internal jugular vein in four patients, and via the femoral vein in the rest.
Evolution of CAVB
In the majority of group A patients, the temporary pacemaker was removed after 48 h, when sinus rhythm and 1:1 conduction had been recovered and maintained for at least 24 h. No patient required a permanent pacemaker. No significant difference was seen in the time to the recovery of sinus rhythm between the patients who showed hemodynamic deterioration with CAVB and those who tolerated the block better. In group B, the long duration of CAVB (in 5 patients it lasted more than 4 days), required the electrocatheter to be maintained in place for longer (in 1 patient for 10 days). One patient required the implantation of a permanent pacemaker.
In 1 of the group A patients who presented with ventricular tachycardia (VT), ventricular fibrillation (VF) occurred during the implantation of the provisional electrocatheter. This was probably associated with the procedure since its onset coincided with the moment when contact was made between the electrode and the tricuspid valve. Electrical defibrillation (200 J) was effective and the patient showed no clinical sequelae. Two group A patients (11.7%) showed a large femoral hematoma that necessitated a blood transfusion due to a greater than 10 point fall from the baseline hematocrit value. In both cases the hematoma was resolved with conservative treatment without the need for surgical drainage. None of the group B patients experienced complications due to the implantation of the provisional pacemaker (P=NS).
The in-hospital mortality was 12.8% (n=5) in group A and 46% (n=6; P=.03) in group B. In group A, 3 patients died of refractory cardiogenic shock and two of electromechanical dissociation. All were in sinus rhythm at the moment of death. In group B, 4 patients died of refractory cardiogenic shock (all with unresolved CAVB at the moment of death), 1 due to VF that degenerated into asystole, and 1 of septic shock due to acute peritonitis (after having recovered sinus rhythm).
DISCUSSION
A number of studies have analyzed the incidence of CAVB and its effect on the prognosis of patients with inferior wall AMI, both in the pre-thrombolytic era5 and later when fibrinolysis had become a standard procedure.1-9,14 However, little information is available on the characteristics and duration of CAVB in patients receiving fibrinolytic treatment, or on the therapeutic approach to be followed in these cases. Until now, the work of McNeaill et al8 involved the largest group of patients (n=21). In the present work, however, group A was comprised of 39 patients, all with inferior wall AMI complicated by CAVB. This was appreciable in the initial ECG of 43% of these patients. The majority received fibrinolytic treatment within two hours of onset. Similar presentation data have been recorded in other studies,1,6,8 including the TIMI II study1, in which 54% of patients presented with CAVB at admission, and the study by Melgarejo et al,6 in which 67% of patients showed CAVB at admission or within the next hour.
Although the incidence of CAVB was similar in both the present groups it was significantly shorter lasting in those patients who received fibrinolysis (group A) than in those who did not (group B) (median 75 min compared to 1440 min respectively; P=.004). Despite the small number of patients in this study, this finding is of clinical interest since it supports the idea that thrombolysis should be attempted in patients with inferior wall AMI plus CAVB as a means of quickly resolving this complication. Indeed, if rapid resolution can be expected, the use of more aggressive techniques such as the implantation of a provisional electrocatheter (which is not free of risk) might be avoided.
In the present study, once the fibrinolytic treatment had been administered, the CAVB reverted rapidly and sinus rhythm and 1:1 conduction were restored in little more than an hour in around half of the patients, and in under two hours in 69%. These data agree with those of McNeaill et al,8 who reported the reversion of CAVB in 52% of patients within the first 2 hours of initiating thrombolysis, and with those of the TAMI study,14 which reported CAVB to last a mean of 2.5 h and under 12 h in 75% of patients.
In the present patients, the administration of atropine (a class I indication in the Sociedad Española de Cardiología guidelines)15 achieved a reversion of CAVB and 1:1 conduction in 16.6% of group A patients, and in 33% of group B patients. These are not inappreciable results given the few adverse effects associated with such treatment; in our opinion, atropine treatment should always be initially attempted unless there are clear contraindications. The administration of dopamine or isoproterenol is not contemplated in the above guidelines, nor is their use mentioned in other studies. In the present work, dopamine was administered to only a small number of patients, but sinus rhythm was achieved in 66% of those in group A. This drug might be of use in inferior wall AMI with symptomatic CAVB, especially at centers where provisional pacemakers cannot be implanted. Dopamine and isoproterenol can increase the frequency of the ventricular escape rhythm and induce mild clinical and hemodynamic improvement. However, the results of the present and other studies8 show that they only rarely revert CAVB on their own.
In the majority of patients who received fibrinolysis, CAVB was relatively well tolerated. Only 30% of the present patients showed significant hemodynamic deterioration (hypotension and severe bradycardia) with no response to atropine. These patients received a provisional electrocatheter, and their clinical situations improved with the increase in heart rate induced by the pacemaker.
The efficacy of fibrinolysis as a treatment for inferior wall AMI has been demonstrated,10,11,16 the benefits being greater the earlier this treatment begins.12,13 The implantation of a provisional electrocatheter could cause a delay in starting fibrinolytic treatment--a delay could be longer for patients initially treated at small hospitals who are then referred to larger centers for a provisional pacemaker to be implanted. Thrombolytic treatment is probably the most efficient in the reversion of CAVB17; tardy initiation could delay the reperfusion of the atrioventricular node, the ischemia of which could, hypothetically, be related to the physiology of CAVB.1 As a consequence the duration of CAVB would be longer.
In the present study, provisional pacemakers were implanted in 43% of group A patients (17 out of 39). However, the number so treated differs widely between similar studies, e.g., in the study performed by Melgarejo et al,6 58% of patients received a temporary pacemake r, whereas only 17% received the same in the study performed by McNeaill et al.8 Twelve of our 17 group A patients (30%) showed severe bradycardia and hypotension, which suggests the implantation of an electrocatheter was advisable. However, the results of this study show that such a course of treatment for the other 5 is questionable. The implantation of a provisional pacemaker was required more often in group B patients, who received no thrombolytic treatment (84.6% compared to 43%; P=.01). In this group, a large majority showed considerable hemodynamic deterioration (hypotension and severe bradycardia), and the duration of CAVB was significantly longer.
Transvenous transient cardiac stimulation in a patient who has received, or is shortly to receive fibrinolytic treatment, is not free of complications. In the present study, 2 patients (11.8%) developed a femoral hematoma (resolved with local pressure) that necessitated a blood transfusion. McNeaill et al8 did not report this complication, although Melgarejo et al6 reported 3.6% of their patients to suffer the same. Although the maximum thrombolytic effect does not coincide with the implantation of the electrocatheter, the risk of hemorrhage persists for some 24-48 h after the procedure, owing to the effects of coadjuvant therapy with heparin and anti-aggregation drugs.
The implantation of a provisional electrocatheter is also associated with a risk of potentially lethal arrhythmias in about 3.6% of cases.6 In group A, 1 patient (5.8%) had a VT that degenerated into VF during the implantation of the electrocatheter; this occurred when the electrode came into contact with the tricuspid valve. The situation was resolved with defibrillation. There is also a risk that the electrocatheter might perforate the ventricle. Some studies estimate this to occur in 4% of cases,18 but it could be more common in patients with an AMI involving the right ventricle since the tissue is less robust. No patient suffered this complication in the present study, nor were any so affected in the studies of Melgarejo et al6 or McNeaill et al.8
An alternative to the transvenous electrocatheter procedure is to use a transcutaneous pacemaker. However, this is a very painful technique and generally requires intense analgesia, perhaps even pharmacological sedation, which could cause the patient to deteriorate. It should therefore only be used when there is hemodynamic deterioration (hypotension and severe bradycardia), and when there is insufficient infrastructure to allow the immediate implantation of a provisional, transvenous electrocatheter.
Limitations of the Study
The present work is an observational study in which the control group is small (patients admitted with an AMI involving the inferior wall within the last 6 h, complicated by CAVB, and who received no fibrinolytic treatment). Over the 10 year study period, no further patients who met the inclusion criteria were available at our hospital; since the year 2000, all AMI patients have received thrombolysis or angioplasty within the first 6 h of an AMI unless clearly contraindicated. Even so, the study is of clinical interest since it provides information on the duration of CAVB in patients who are candidates for fibrinolysis--it is these cases that present the greatest doubts about which therapeutic course to follow. However, the very fact that significant results were obtained even though the numbers were small, underlines the value of the conclusions.
CLINICAL IMPLICATIONS
Given the present results, and those of other authors, the duration of CAVB in inferior wall AMI is brief when treated by fibrinolysis. This complication lasts a significantly shorter length of time when thrombolysis is performed than when no reperfusion therapy is followed. The mortality associated with the above condition is also lower when such treatment is provided. Thus, fibrinolytic treatment should be provided early in CAVB.
In the present study, thrombolytic treatment led to fewer patients requiring a provisional pacemaker. Given the short duration of CAVB, transitory stimulation of the heart via intravenous access should be reserved for patients with poor hemodynamic tolerance (patients with CAVB and low ventricular frequencies, hypotension, and/or cardiogenic shock), since this technique is not free of complications.
ACKNOWLEDGEMENTS
The authors thank Drs. Josep Lupón and Jorge López Ayerbe of our center for their help with the statistical analysis.
Correspondence: Dr. C. García García.
Servicio de Cardiología. Hospital Universitari Germans Trias i Pujol.
Ctra. del Canyet, s/n. 08916 Badalona. Barcelona. España.
E-mail: cosmegarcia@mixmail.com.