Urinary sodium (UNa+) has emerged as a useful biomarker of poor clinical outcomes in acute heart failure (AHF). Here, we sought to evaluate: a) the usefulness of a single early determination of UNa+ for predicting adverse outcomes in patients with AHF and renal dysfunction, and b) whether the change in UNa+ at 24hours (ΔUNa24h) adds any additional prognostic information over baseline values.
MethodsThis is a post-hoc analysis of a multicenter, open-label, randomized clinical trial (IMPROVE-HF) (ClinicalTrials.gov NCT02643147) that randomized 160 patients with AHF and renal dysfunction on admission to a) the standard diuretic strategy, or b) a carbohydrate antigen 125-guided diuretic strategy. The primary end point was all-cause mortality and total all-cause readmissions.
ResultsThe mean age was 78±8 years, and the mean glomerular filtration rate was 34.0±8.5mL/min/1.73 m2. The median UNa+ was 90 (65-111) mmol/L. At a median follow-up of 1.73 years [interquartile range, 0.48-2.35], 83 deaths (51.9%) were registered, as well as 263 all-cause readmissions in 110 patients. UNa+ was independently associated with mortality (HR, 0.75; 95%CI, 0.65-0.87; P <.001) and all-cause readmissions (HR, 0.92; 95%CI, 0.88-0.96; P <.001). The prognostic usefulness of the ΔUNa24h varied according to UNa+ at admission (P for interaction <.05). The ΔUNa24h was inversely associated with both end points only in the group with UNa+ ≤ 50 mmol/L. Conversely, no effect was found in the group with UNa+> 50 mmol/L.
ConclusionsIn patients with AHF and renal dysfunction, a single early determination of UNa+ ≤ 50 mmol/L identifies patients with a higher risk of all-cause mortality and readmission. The ΔUNa24h adds prognostic information over baseline values only when UNa+ at admission is ≤ 50 mmol/L.
Keywords
Intravenous loop diuretics are the cornerstone of the treatment of acute heart failure (AHF) syndromes.1 Nonetheless, diuretic dose titration and decongestion monitoring remain an unsolved clinical dilemma, particularly given the heterogeneity in volume overload.1 Current decongestion monitoring practice largely relies on serial weight changes, clinical examination, and net fluid loss. However, these metrics offer a modest ability for assessing the adequacy of the treatment response.2 A relatively recent alternative biomarker to identify patients at risk of diuretic resistance and worse clinical outcomes is urinary sodium content (UNa+).3–7 Thus, it seems plausible to speculate that serial assessment of UNa+ during decompensation would have a clinically useful role in assessing the patient response to diuretics.8 Indeed, in patients with AHF, a recent position statement suggested the use of serial UNa+ assessment during the early course of decompensation to monitor responses and tailor the intensity of the diuretic treatment.1 However, data endorsing the clinical usefulness of early UNa+ serial measurement are scarce or even nonexistent in patients who concomitantly display renal dysfunction, a condition that may greatly influence the excretion of UNa+.
In this work, we sought to evaluate: a) the clinical usefulness of a single determination of spot UNa+ at presentation for predicting all-cause mortality and total readmissions in patients with AHF and renal dysfunction on admission, and b) whether the change in UNa+ at 24hours (ΔUNa24h) provides any additional prognostic advantage over baseline UNa+ values.
METHODSStudy populationThis study is a post-hoc analysis of a multicenter, open-label, parallel randomized controlled trial (IMPROVE-HF). In that trial, patients with AHF and renal dysfunction at presentation were 1:1 randomized to a) a standard loop diuretics dosage based on routine clinical evaluation, or b) carbohydrate antigen 125 (CA125)-guided therapy. The inclusion and exclusion criteria were published previously2 and are presented in . This study was registered with ClinicalTrials.gov (NCT02643147). A detailed description of the trial design is presented elsewhere.2,9
The study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice and fully conformed to national regulations. The protocol, informed consent form, participant information sheet, and all applicable documents were approved by the appropriate Ethics Committee (Comite de Ética del Hospital Clínico Universitario de Valencia) and by the Agencia Española del Medicamento y Productos Sanitarios (AEMPS). All patients provided written informed consent. All analyses were performed by an independent company (MedStats Consulting, United States).
ProceduresOnce the diagnosis of AHF was confirmed, patients were screened and randomized within the first 24hours. During this visit, clinical characteristics and biomarkers were assessed. Scheduled follow-up visits were performed at 24hours, 72hours, and 30 days after randomization (final visit).
Eligible patients were randomized to receive intravenous diuretics with the dosage based on either conventional clinical evaluation or on CA125 values. In the conventional clinical evaluation arm, the diuretic strategy was based on the presence of signs and symptoms of systemic congestion according to current guideline recommendations.10 In the active arm, higher diuretic doses were recommended in patients with CA125> 35 U/mL. In contrast, lower doses were recommended when CA125 ≤ 35 U/mL. The diuretic strategies in both arms are summarized in .
UNa+ assessmentUNa+ was measured at patient randomization and at 24hours. The mean time from admission to randomization was 6±3hours, and the median and interquartile range [IQR] dose of intravenous furosemide received before randomization was 40 [20-60] mg. Based on previous studies,3,8,11 UNa+ at admission was dichotomized at 50 mmol/L (≤ 50 vs> 50 mmol/L).
Follow-up and end pointsThe primary end point was to assess whether a single determination of UNa+ at admission predicts all-cause mortality and all-cause readmission rates. In addition, we sought to evaluate if changes in UNa+ at 24hours (ΔUNa24h) provided any additional prognostic advantage over baseline UNa+ values. For this purpose, the ΔUNa24h was tested against clinical end points at UNa+ ≤ 50 and> 50 mmol/L (UNa+50). Ambulatory follow-up was performed in the HF units of each center. End points were adjudicated by reviewing the electronic discharge records of our regional health care system. Only unplanned readmissions were included. HF-related readmissions were those with worsening or acute HF as the main diagnosis at discharge. The researchers in charge of end point adjudications were all blinded to the exposure.
Statistical analysisBaseline continuous characteristics according to UNa+50 are presented as mean±standard deviation and median [interquartile range] as appropriate; categorical variables are presented as frequencies with percentages. Continuous variables were compared according to UNa+50 with either the t test or rank-sum test for independent samples depending on the variable distribution. Discrete variables were compared using the chi-square test.
Variable selection for regression modelsCandidate covariates were chosen based on previous medical knowledge, independently of their P values. A reduced and parsimonious model was derived using backward elimination. During this selection process, the linearity assumption for continuous variables was tested and transformed, if appropriate, with fractional polynomials.12
In all analyses, the hospital center was included as a stratification factor, and the randomization variable from the original randomized controlled trial was forced as a covariate.
Association with clinical end pointsA bivariate negative binomial regression was used to determine the direction and strength of the association of exposures (UNa+ and interaction of the UNa+50 with the ΔUNa24h) with all-cause readmissions and all-cause mortality. Coefficients from this method are estimated by accounting for the positive correlation among the recurrent outcome and death as a terminal event by linking the 2 simultaneous equations (readmission count and death) with shared frailty.13 In addition, each patient's follow-up time was used as an offset in the models to account for differences in the follow-up. In the end, by using this methodology, the potential for bias due to death as informative censoring is minimized, an issue commonly seen in AHF studies. Risk estimates from this method are presented as incidence rate ratios (IRRs) and 95% confidence intervals (95%CIs). The covariates included in each model are presented with the corresponding figure legends. Completeness of follow-up was calculated with the Clark or completeness index C (95.3%).
The shape and direction of the ΔUNa24h association trajectory (as IRRs) was depicted along its continuum and stratified by UNa+ status (UNa+ ≤ 50 mmol/L vs UNa+> 50 mmol/L). A portion of the trajectory above or below 1 in the y-axis was deemed to be significant.
A 2-sided P value of <.05 was set as the threshold for statistical significance. Stata 15.1 (Stata Statistical Software, Release 15 [2017]; StataCorp LP, United States) was used for the primary analysis. Risk reclassification analyses (survIDINRI and SurvC1 modules) were implemented in R (version 3.5.2; R Foundation for Statistical Computing, Austria).
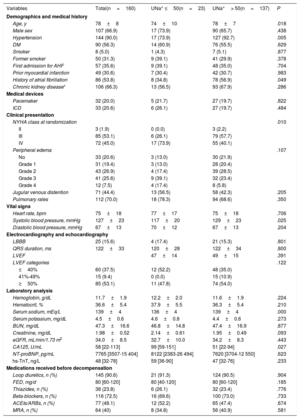
RESULTSPatientsA total of 160 patients were included in this study between March 2015 and December 2016 at 9 centers in Spain. The mean age of the study population was 78±8 years, 66.9% were male, and 46.9% had left ventricular ejection fraction <50%. The median [IQR, p25-p75] levels of N-terminal pro-brain natriuretic peptide and CA125 were 7765 pg/mL [3507-15404] and 58 U/mL [22-113], respectively. The mean BUN and creatinine levels and estimated glomerular filtration rate (eGFR) were 47.3±16.6mg/dL, 1.98±0.52mg/dL, and 34±8.5mL/min/1.73 m2, respectively. By design, all patients had renal dysfunction (eGFR <60mL/min/1.73 m2), and 50 patients (31.3%) had an eGFR <30mL/min/1.73 m2 at admission. Chronic kidney disease (eGFR <60mL/min/1.73 m2 using serum creatinine obtained at the latest available outpatient visit prior to admission and assessed during a stable phase of the disease) was present in 106 patients (66.3%). All patients received intravenous furosemide on admission, and the median [IQR] dose was 40 [20-60] mg. The accumulated doses at 24 and 72hours postrandomization were 190 [120-320] and 410 [250-640], respectively. Detailed characteristics of the study sample are presented in table 1.
Baseline characteristics according to urinary sodium content at admission
| Variables | Total(n=160) | UNa+ ≤50(n=23) | UNa+> 50(n=137) | P |
|---|---|---|---|---|
| Demographics and medical history | ||||
| Age, y | 78±8 | 74±10 | 78±7 | .018 |
| Male sex | 107 (66.9) | 17 (73.9) | 90 (65.7) | .438 |
| Hypertension | 144 (90.0) | 17 (73.9) | 127 (92.7) | .005 |
| DM | 90 (56.3) | 14 (60.9) | 76 (55.5) | .629 |
| Smoker | 8 (5.0) | 1 (4.3) | 7 (5.1) | .877 |
| Former smoker | 50 (31.3) | 9 (39.1) | 41 (29.9) | .378 |
| First admission for AHF | 57 (35.6) | 9 (39.1) | 48 (35.0) | .704 |
| Prior myocardial infarction | 49 (30.6) | 7 (30.4) | 42 (30.7) | .983 |
| History of atrial fibrillation | 86 (53.8) | 8 (34.8) | 78 (56.9) | .049 |
| Chronic kidney disease* | 106 (66.3) | 13 (56.5) | 93 (67.9) | .286 |
| Medical devices | ||||
| Pacemaker | 32 (20.0) | 5 (21.7) | 27 (19.7) | .822 |
| ICD | 33 (20.6) | 6 (26.1) | 27 (19.7) | .484 |
| Clinical presentation | ||||
| NYHA class at randomization | .010 | |||
| II | 3 (1.9) | 0 (0.0) | 3 (2.2) | |
| III | 85 (53.1) | 6 (26.1) | 79 (57.7) | |
| IV | 72 (45.0) | 17 (73.9) | 55 (40.1) | |
| Peripheral edema | .107 | |||
| No | 33 (20.6) | 3 (13.0) | 30 (21.9) | |
| Grade 1 | 31 (19.4) | 3 (13.0) | 28 (20.4) | |
| Grade 2 | 43 (26.9) | 4 (17.4) | 39 (28.5) | |
| Grade 3 | 41 (25.6) | 9 (39.1) | 32 (23.4) | |
| Grade 4 | 12 (7.5) | 4 (17.4) | 8 (5.8) | |
| Jugular venous distention | 71 (44.4) | 13 (56.5) | 58 (42.3) | .205 |
| Pulmonary rales | 112 (70.0) | 18 (78.3) | 94 (68.6) | .350 |
| Vital signs | ||||
| Heart rate, bpm | 75±18 | 77±17 | 75±18 | .706 |
| Systolic blood pressure, mmHg | 127±23 | 117±20 | 129±23 | .025 |
| Diastolic blood pressure, mmHg | 67±13 | 70±12 | 67±13 | .204 |
| Electrocardiography and echocardiography | ||||
| LBBB | 25 (15.6) | 4 (17.4) | 21 (15.3) | .801 |
| QRS duration, ms | 122±33 | 120±28 | 122±34 | .800 |
| LVEF | 47±14 | 49±15 | .391 | |
| LVEF categories | .122 | |||
| ≤40% | 60 (37.5) | 12 (52.2) | 48 (35.0) | |
| 41%-49% | 15 (9.4) | 0 (0.0) | 15 (10.9) | |
| ≥50% | 85 (53.1) | 11 (47.8) | 74 (54.0) | |
| Laboratory analysis | ||||
| Hemoglobin, g/dL | 11.7±1.9 | 12.2±2.0 | 11.6±1.9 | .224 |
| Hematocrit, % | 36.6±5.4 | 37.9±5.5 | 36.3±5.4 | .210 |
| Serum sodium, mEq/L | 139±4 | 136±4 | 139±4 | .000 |
| Serum potassium, mg/dL | 4.5±0.6 | 4.6±0.8 | 4.4±0.6 | .273 |
| BUN, mg/dL | 47.3±16.6 | 46.8±14.8 | 47.4±16.9 | .877 |
| Creatinine, mg/dL | 1.98±0.52 | 2.14±0.61 | 1.95±0.49 | .093 |
| eGFR, mL/min/1.73 m2 | 34.0±8.5 | 32.7±10.0 | 34.2±8.3 | .443 |
| CA125, U/mL | 58 [22-113] | 99 [59-151] | 51 [22-94] | .027 |
| NT-proBNP, pg/mL | 7765 [3507-15 404] | 8122 [2383-26 494] | 7620 [3704-12 550] | .623 |
| hs-TnT, ng/L | 48 [32-78] | 59 [36-90] | 47 [32-76] | .233 |
| Medications received before decompensation | ||||
| Loop diuretics, n (%) | 145 (90.6) | 21 (91.3) | 124 (90.5) | .904 |
| FED, mg/d | 80 [60-120] | 80 [40-120] | 80 [60-120] | .185 |
| Thiazides, n (%) | 38 (23.8) | 6 (26.1) | 32 (23.4) | .776 |
| Beta-blockers, n (%) | 116 (72.5) | 16 (69.6) | 100 (73.0) | .733 |
| ACEIs/ARBs, n (%) | 77 (48.1) | 12 (52.2) | 65 (47.4) | .674 |
| MRA, n (%) | 64 (40) | 8 (34.8) | 56 (40.9) | .581 |
ACEIs, angiotensin converting enzyme inhibitors; AHF, acute heart failure; ARBs, angiotensin II receptor blockers; BUN, blood urea nitrogen; CA125, antigen carbohydrate 125; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FED, furosemide equivalent dose; hs-TnT, high-sensitivity troponin T; ICD, implantable cardioverter-defibrillator; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, amino-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; QRS, QRS interval; UNa+, urinary sodium content.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
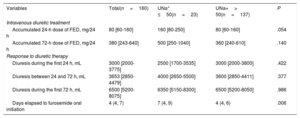
The median [IQR] of UNa+ content at randomization was 90 mmol/L [65-111], and UNa+ ≤ 50 mmol/L was present in 23 patients (14.4%). Patients with UNa+ ≤ 50 mmol/L were younger, exhibited worse New York Heart Association (NYHA) class at randomization, and had higher baseline levels of CA125. Nevertheless, no significant differences were found in natriuretic peptide values or in clinical signs of congestion vs those with UNa+> 50 mmol/L (table 1). Moreover, the 2 groups were balanced regarding prior diuretic treatment (table 1). Intravenous diuretic therapy according to UNa+50 is presented in table 2. There was a statistical trend for a higher intravenous furosemide equivalent dose (FED) during the first 24hours in patients with UNa+ ≤ 50 mmol/L. No differences were found at 72hours. Accumulated diuresis during the first 24 and 72hours did not significantly differ according to UNa+50. Time to cessation of intravenous furosemide was longer in patients with UNa+ ≤ 50 mmol/L (table 2).
Intravenous diuretic treatment and response to diuretic therapy
| Variables | Total(n=160) | UNa+ ≤50(n=23) | UNa+> 50(n=137) | P |
|---|---|---|---|---|
| Intravenous diuretic treatment | ||||
| Accumulated 24-h dose of FED, mg/24 h | 80 [60-160] | 160 [80-250] | 80 [60-160] | .054 |
| Accumulated 72-h dose of FED, mg/24 h | 380 [243-640] | 500 [250-1040] | 360 [240-610] | .140 |
| Response to diuretic therapy | ||||
| Diuresis during the first 24 h, mL | 3000 [2000-3775] | 2500 [1700-3535] | 3000 [2000-3800] | .422 |
| Diuresis between 24 and 72 h, mL | 3653 [2850-4479] | 4000 [2650-5500] | 3600 [2850-4411] | .377 |
| Diuresis during the first 72 h, mL | 6500 [5200-8075] | 6350 [5150-8300] | 6500 [5200-8050] | .986 |
| Days elapsed to furosemide oral initiation | 4 (4, 7) | 7 (4, 9) | 4 (4, 6) | .006 |
FED, furosemide equivalent dose; UNa+, urinary sodium content.
Data are expressed as No. (%), or median [interquartile range].
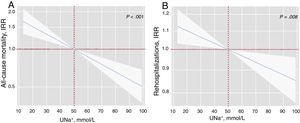
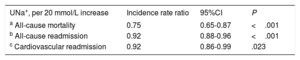
After a median follow-up of 1.73 [0.48-2.35] years, 83 all-cause deaths (51.9%) were recorded: 51 (61.45%) corresponded to HF-related deaths, 8 (9.64%) to other cardiovascular causes, and 24 (28.92%) to noncardiovascular deaths. In a multivariable setting, UNa+ was significantly, inversely, and linearly associated with a higher risk of mortality (figure 1A). Indeed, for each 20-mmol/L increase in UNa+, there was a 25% decrease in the incidence of mortality (table 3). A sensitivity analysis revealed that UNa+ was also inversely and independently associated with a higher risk of HF- and cardiovascular-related death (.
Early spot UNa+ and risk of adverse clinical outcomes. A, early spot UNa+ and long-term all-cause mortalitya. B, early spot UNa+ and long-term total readmissionsb. a Model adjusted by hospital center, age, sex, randomization variable, prior admission for acute heart failure, ischemic heart disease, systolic blood pressure, glomerular filtration rate, blood urea nitrogen, N-terminal pro-brain natriuretic peptide, and furosemide equivalent dose prior to randomization (mg/24h). b Model adjusted by hospital center, randomization variable, prior admission for acute heart failure, ischemic heart disease, systolic blood pressure, glomerular filtration rate, blood urea nitrogen, N-terminal pro-brain natriuretic peptide, hemoglobin, left ventricular ejection fraction, and furosemide equivalent dose prior to randomization (mg/24h). IRR, incidence rate ratio; UNa+, urinary sodium.
Clinical events by spot urinary sodium
| UNa+, per 20 mmol/L increase | Incidence rate ratio | 95%CI | P |
|---|---|---|---|
| a All-cause mortality | 0.75 | 0.65-0.87 | <.001 |
| b All-cause readmission | 0.92 | 0.88-0.96 | <.001 |
| c Cardiovascular readmission | 0.92 | 0.86-0.99 | .023 |
95%CI, 95% confidence interval; UNa+, urinary sodium.
Model covariates: hospital center, age, sex, randomization variable, prior admission for acute heart failure, ischemic heart disease, systolic blood pressure, glomerular filtration rate, blood urea nitrogen, N-terminal pro-brain natriuretic peptide, and furosemide equivalent dose prior to randomization (mg/24h).
Model covariates: hospital center, randomization variable, prior admission for acute heart failure, ischemic heart disease, systolic blood pressure, glomerular filtration rate, blood urea nitrogen, N-terminal pro-brain natriuretic peptide, hemoglobin, left ventricular ejection fraction, and furosemide equivalent dose prior to randomization (mg/24h).
Model covariates: hospital center, randomization variable, prior admission for acute heart failure, ischemic heart disease, systolic blood pressure, glomerular filtration rate, blood urea nitrogen, N-terminal pro-brain natriuretic peptide, left ventricular ejection fraction, and furosemide equivalent dose prior to randomization (mg/24h).
During follow-up, 263 all-cause readmissions were recorded in 110 patients, as well as 160 HF readmissions in 81 patients. The distribution of all-cause readmissions per patient was 1, 2, 3, and> 3 in 45, 24, 18, and 23 patients, respectively. For HF readmissions, the distribution per patient was 1, 2, 3, and> 3 in 40, 24, 6, and 11 patients, respectively.
As shown in figure 1B, UNa+ was independently and inversely associated with the risk of all-cause readmissions. For every 20-mmol/L increase along its continuum, there was an associated 8% decrease in all-cause readmissions (table 3). Likewise, UNa+ was inversely associated with the risk of recurrent HF readmission (table 3).
Additional prognostic value of ΔUNa24hThe mean eGFR was significantly increased at 24hours (34.0±8.5 vs 35.4±8.5mL/min/1.73 m2, P=.004). Likewise, the mean UNa+ was decreased vs baseline [median delta change of−5.5 (−25 to 12.6) mmol/L]. In total, 98 patients (61.3%) had a decreased/unchanged ΔUNa24h. Patients with a decreased/unchanged ΔUNa24h showed a trend for higher rates of death (3.9 vs 2.6 per 10 person-years, P=.094) but not for all-cause readmissions (17.9 vs 14.0 per 10 person-years, P=.279).
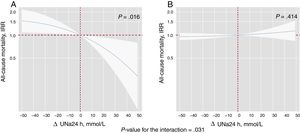
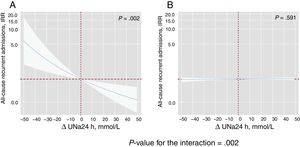
By modeling the effect of the ΔUNa24h on the risk of mortality and readmissions, we found a significant interaction with baseline values of UNa+50. The ΔUNa24h was inversely associated with all-cause mortality only in the group with UNa+ ≤ 50 mmol/L. Conversely, no effect was found in the group with UNa+> 50 mmol/L (figure 2). The same differential effect was found for total readmissions (figure 3). The interactions between ΔUNa24h and baseline UNa+ were no longer significant when the baseline values of UNa+ were dichotomized at 60 mmol/L (P value for interaction=.080) and 70 mmol/L (P value for interaction=.837).
Differential effects of ΔUNa24h on all-cause mortality according to UNa+ at baseline. IRR depiction along the continuum of the ΔUNa24h according to the 2 levels of UNa+ at baseline. A, UNa+ at baseline ≤ 50 mmol/L. B, UNa+ at baseline> 50 mmol/L. ΔNaU24h modeled with fractional polynomial [3] and centered at the “0” value. The omnibus P value for the interaction, P=.031. The analysis was adjusted by hospital center, randomization variable, age, sex, systolic blood pressure, glomerular filtration rate, N-terminal pro-brain natriuretic peptide, urine volume at 24hours, and FED at 24hours. ΔUNa24h, change in UNa+ at 24hours; FED, furosemide equivalent dose; IRR, incidence rate ratio; UNa+, urinary sodium.
Differential effects of ΔUNa24h on all-cause recurrent admissions according to UNa+ at baseline. IRR depiction along the continuum of the ΔUNa24h according to the 2 levels of UNa+ at baseline. A, UNa+ at baseline ≤ 50 mmol/L. B, UNa+ at baseline> 50 mmol/L. ΔUNa24h modeled with fractional polynomial [0] and centered at the “0” value. The omnibus P value for the interaction, P=.002. Analysis adjusted by hospital center, randomization variable, prior admission for heart failure, ischemic etiology, systolic blood pressure, glomerular filtration rate, blood urea nitrogen, hemoglobin, N-terminal pro-brain natriuretic peptide, urine volume at 24hours, and FED at 24hours. ΔUNa24h, change in UNa+ at 24hours; FED, furosemide equivalent dose; IRR, incidence rate ratio; UNa+, urinary sodium.
The primary findings of the present study in patients with AHF and concomitant renal dysfunction confirm the value of the early assessment of UNa+ for predicting all-cause mortality. In addition, to the best of our knowledge, this is the first work endorsing the association between low UNa+ and a higher burden of rehospitalization. Notably, the risk estimates were adjusted for established prognosticators and prior diuretic regimen. Interestingly, we found that the trajectory of UNa+ excretion after 24hours of diuretic therapy provides additional prognostic information over baseline values only in the subgroup of patients with lower UNa+ content at baseline (absolute UNa+ ≤ 50 mmol/L). Taken together, these findings suggest that a single measurement of UNa+ at admission may be enough to identify patients at high risk of adverse events. UNa+ monitoring during hospitalization might provide useful clinical information, particularly in patients with lower values at admission.
Prognostic value of spot UNa+UNa+ has emerged as a promising biomarker in HF.3–7 From a pathophysiological point of view, lower UNa+ may result from a decrease in the glomerular filtration rate.14 However, most of the evidence indicates that lower UNa+ mainly identifies patients with renal tubular dysregulation secondary to sustained neurohormonal activation.15,16 Thus, lower UNa+ has been shown to identify patients at higher risk of adverse events and diuretic resistance.3–7 This study confirms these findings in patients with AHF and concomitant renal dysfunction. In agreement with recent work, we found that early assessment of UNa+ is strongly associated with the risk of long-term mortality, despite a thorough patient adjustment. Interestingly, we also found a significant and adjusted association of spot UNa+ with the burden of total hospitalizations in this high-risk population that enrolled a subset of patients with severe renal dysfunction at presentation.
Although we cannot compare the discriminative accuracy of spot UNa+ at admission vs UNa24h, we believe that spot determinations offer important advantages over UNa24h. First, the spot assessment might provide early clinical information with potential prognostic and therapeutic implications. Second, spot UNa+ at admission or during the early course of admission is much easier to implement in real-world clinical practice.
Prognostic value of the early UNa+ trajectory in AHFSerial assessment of UNa+ during hospitalization for AHF has been suggested to be useful for monitoring the diuretic response and eventually guiding the intensity of depletive treatment. Indeed, a recent position statement from the Heart Failure Association of the European Society of Cardiology (ESC-HF) recommended UNa+ measurement as part of a stepped pharmacological care algorithm1 in patients with AHF and congestion. According to that document, a spot UNa+ <50–70 mmol/L 2hours after the starting dose of loop diuretics should alert clinicians to the risk of diuretic resistance and motivate prompt interventions.1 Although this “tubular stress test” is plausible and attractive, there is little evidence supporting the clinical role of UNa+ serial measurement in patients with AHF syndromes. Biegus et al.8 recently reported that longitudinal spot UNa+ analysis during consecutive days of decongestion provides further clinical and prognostic information. They showed that patients with decreased/unchanged UNa+ excretion at 48hours had less effective decongestion, poorer diuretic efficacy, and higher risk of 1-year mortality. However, several aspects should be acknowledged when interpreting longitudinal UNa+ dynamics. First, urinary composition is characterized by a progressive decline in UNa+ along the course of diuretic therapy.7 Once extracellular volume overload has been reduced, natriuresis also declines to allow sodium excretion to once again equal intake.17 Although this phenomenon is frequently maladaptive in AHF (sodium retention in the setting of persistent congestion), it might be difficult to ascertain whether the observed decline in UNa+ indicates diuretic resistance or, in contrast, represents a physiological response to extracellular volume contraction. In addition, and regardless of the prognostic usefulness, we already envision that, in the absence of specific and well-established treatment implications, it may be difficult to widely implement serial assessment in clinical practice. However, we should also note that isolated assessment of UNa+ in the first hours or days of decompensation also provides useful prognostic information, as reported here and in other studies.8,18,19
According to the present findings, early changes in UNa+ may provide meaningful prognostic information in patients with lower UNa+ values at baseline. This subset of patients identified a subgroup with ominous prognosis whose UNa+ monitoring may provide useful clinical information on the treatment response. In contrast, in the presence of higher values at baseline (UNa+> 50 mmol/L), early UNa+ changes may not provide additional information to better define patient risk. Further research is required to confirm the present findings and, more importantly, elucidate the role of UNa+ as a therapeutic target.
Several limitations to our study need to be acknowledged. First, this was a post-hoc analysis of the IMPROVE-HF trial, which was not designed to evaluate the prognostic value of UNa+ in AHF. Therefore, all of the results should be considered exploratory and hypothesis-generating. Second, due to the limited sample size, some of the negative results could be explained by type II error (insufficient statistical power). Third, the IMPROVE-HF cohort was primarily composed of patients with AHF and concomitant renal dysfunction. Therefore, it is unclear how the results will apply to the broader AHF population. In addition, with the present data, we cannot accurately distinguish between patients with acute vs chronic renal dysfunction at presentation. Fourth, although patients were recruited in 9 centers in Spain and the follow-up was performed by a multidisciplinary team (cardiologists and internal medicine specialists), it is unknown how these results will apply to other health care systems with a different HF management organization.20 Fifth, we did not evaluate the effect of UNa+ on other surrogates of decongestion, such as weight and venous pressures. Sixth, we cannot account for differences in sodium intake before admission or during decompensation. As such, we cannot evaluate its effect as a confounder. Seventh, we used spot urine samples and not 24-hour urine collection. Therefore, we cannot compare the prognostic value of spot vs 24-hour collection. Eighth, baseline UNa+ is potentially confounded by the timing of measurement and the last administration of intravenous diuretics. This period was not recorded in this study. Lastly, the threshold for the identification of a poor natriuretic response remains arbitrary. Further studies should confirm the optimal cutoffs.
CONCLUSIONSIn patients with AHF and renal dysfunction, a single and early determination of UNa+ identifies a subgroup of patients with a higher risk of all-cause mortality and readmissions. ΔUNa24h adds prognostic information over baseline values when UNa+ at presentation is ≤ 50 mmol/L.
- -
Spot UNa+ has emerged as a reliable and widely available prognostic marker in AHF, identifying patients at a higher risk of adverse events.
- -
Increasing evidence suggests that the diuretic and natriuretic response to loop diuretic therapy can be accurately predicted from a spot urine sample collected 1 to 2hours after loop diuretic administration.
- -
This work reveals an independent association between low spot UNa+ at admission and a higher burden of rehospitalization in patients with AHF and concomitant renal dysfunction.
- -
In addition, we suggest that the trajectory of UNa+ excretion after 24hours of diuretic therapy provides additional prognostic information over baseline values, particularly in patients with lower UNa+ content at baseline (absolute UNa+ ≤ 50 mmol).
This work has been funded by Project PI13/01519 in collaboration with Plataforma de Unidades de Investigación Clínica y Ensayos Clínicos (SCReN) (PT13/0002/0031) and cofinanced by Fondos FEDER; by unrestricted grants from Proyectos de Investigación de Insuficiencia Cardiaca de la Sección de Insuficiencia Cardiaca 2015 and Beca Mutual Médica 2014; and by grant numbers PIE15/00013 and CIBER CV 16/11/00420 and 16/11/00403.
CONFLICTS OF INTERESTThe authors declare that they have no conflict of interest.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.06.004


![Differential effects of ΔUNa24h on all-cause mortality according to UNa+ at baseline. IRR depiction along the continuum of the ΔUNa24h according to the 2 levels of UNa+ at baseline. A, UNa+ at baseline ≤ 50 mmol/L. B, UNa+ at baseline> 50 mmol/L. ΔNaU24h modeled with fractional polynomial [3] and centered at the “0” value. The omnibus P value for the interaction, P=.031. The analysis was adjusted by hospital center, randomization variable, age, sex, systolic blood pressure, glomerular filtration rate, N-terminal pro-brain natriuretic peptide, urine volume at 24hours, and FED at 24hours. ΔUNa24h, change in UNa+ at 24hours; FED, furosemide equivalent dose; IRR, incidence rate ratio; UNa+, urinary sodium. Differential effects of ΔUNa24h on all-cause mortality according to UNa+ at baseline. IRR depiction along the continuum of the ΔUNa24h according to the 2 levels of UNa+ at baseline. A, UNa+ at baseline ≤ 50 mmol/L. B, UNa+ at baseline> 50 mmol/L. ΔNaU24h modeled with fractional polynomial [3] and centered at the “0” value. The omnibus P value for the interaction, P=.031. The analysis was adjusted by hospital center, randomization variable, age, sex, systolic blood pressure, glomerular filtration rate, N-terminal pro-brain natriuretic peptide, urine volume at 24hours, and FED at 24hours. ΔUNa24h, change in UNa+ at 24hours; FED, furosemide equivalent dose; IRR, incidence rate ratio; UNa+, urinary sodium.](https://static.elsevier.es/multimedia/18855857/0000007400000007/v1_202106191014/S188558572030253X/v1_202106191014/en/main.assets/thumbnail/gr2.jpeg?xkr=eyJpdiI6IktmaGhwbitwV0NlNlJoWERiS2s1U0E9PSIsInZhbHVlIjoiZnZrOXJDLy8zaDBxazczNkxSSEZhUDVEWGhUaDhsSmJvNFlOclpsMDdCaz0iLCJtYWMiOiIzNzc4N2EyOTMyYWRlZTFkMDdiNjU0NmU2OWZlOTkxODA5ZTU5MjgzOWQwOTQ1YTE2NDAzMzE3ZGUyNGUzMmIxIiwidGFnIjoiIn0=)
![Differential effects of ΔUNa24h on all-cause recurrent admissions according to UNa+ at baseline. IRR depiction along the continuum of the ΔUNa24h according to the 2 levels of UNa+ at baseline. A, UNa+ at baseline ≤ 50 mmol/L. B, UNa+ at baseline> 50 mmol/L. ΔUNa24h modeled with fractional polynomial [0] and centered at the “0” value. The omnibus P value for the interaction, P=.002. Analysis adjusted by hospital center, randomization variable, prior admission for heart failure, ischemic etiology, systolic blood pressure, glomerular filtration rate, blood urea nitrogen, hemoglobin, N-terminal pro-brain natriuretic peptide, urine volume at 24hours, and FED at 24hours. ΔUNa24h, change in UNa+ at 24hours; FED, furosemide equivalent dose; IRR, incidence rate ratio; UNa+, urinary sodium. Differential effects of ΔUNa24h on all-cause recurrent admissions according to UNa+ at baseline. IRR depiction along the continuum of the ΔUNa24h according to the 2 levels of UNa+ at baseline. A, UNa+ at baseline ≤ 50 mmol/L. B, UNa+ at baseline> 50 mmol/L. ΔUNa24h modeled with fractional polynomial [0] and centered at the “0” value. The omnibus P value for the interaction, P=.002. Analysis adjusted by hospital center, randomization variable, prior admission for heart failure, ischemic etiology, systolic blood pressure, glomerular filtration rate, blood urea nitrogen, hemoglobin, N-terminal pro-brain natriuretic peptide, urine volume at 24hours, and FED at 24hours. ΔUNa24h, change in UNa+ at 24hours; FED, furosemide equivalent dose; IRR, incidence rate ratio; UNa+, urinary sodium.](https://static.elsevier.es/multimedia/18855857/0000007400000007/v1_202106191014/S188558572030253X/v1_202106191014/en/main.assets/thumbnail/gr3.jpeg?xkr=eyJpdiI6IjYyenh4b3RvazdhbFlXMzFRNWFCb3c9PSIsInZhbHVlIjoiY0xLYysyV1poMW9oMFc5YUg1b05wQWF4a2lhV2h0eXlMMU0rY2E4VVJ6ST0iLCJtYWMiOiI5NTE4YjhiYjIxM2E1YjdjN2Q3YTEwY2IzMTU2OGMyMWNhMzYyNmM1MGM1MjM5MGRmMDU5NTllYzYxM2Q4YTJjIiwidGFnIjoiIn0=)