In the first cases of coronavirus disease 2019 (COVID-19) described in China, acute myocardial injury was identified as being associated with a worse prognosis.1 The etiology of this myocardial injury is not entirely clear, but it could be related to the processes of microvascular damage, myocarditis, hypoxemia, cytokine-mediated injury, or even stress cardiomyopathy.2,3 However, diagnosis of myocardial injury has mostly been based on raised biomarkers in the absence of cardiac imaging. In this study, we describe the echocardiographic findings of 37 consecutive patients admitted to the intensive care unit (ICU) with acute respiratory distress syndrome secondary to COVID-19.
This was a prospective, single-center study of consecutive patients with COVID-19, confirmed on polymerase chain reaction testing, who were admitted to the ICU due to acute respiratory distress syndrome. The patients were divided into 2 groups based on whether their left ventricular ejection fraction (LVEF) was greater or less than 50%. In patients with reduced function, the severity of the reduction was estimated qualitatively as mild (40%-49%) moderate (30%-39%) or severe (< 30%). Values of high-sensitivity troponin T, N-terminal pro-brain natriuretic peptide, C-reactive protein, and ferritin were considered inflammatory biomarkers, and their peak levels were recorded and compared between the 2 groups. Echocardiography was performed with a handheld ultrasound (Vscan, General Electrics), with visual assessment of right and left ventricular function on 2-, 3-, and 4-chamber views, to minimize patient exposure. The presence of regional wall motion abnormalities, whether they had coronary or noncoronary distribution, and the presence of pericardial effusion were also assessed. Continuous variables are described as median [interquartile range] or mean ± standard deviation and were compared using the Mann-Whitney U test or Student t test depending on the normality of the distribution of the data. Categorical variables are described as percentage and were compared using the Fisher or chi-square test. Data collection was approved by the ethics committee of our institution.
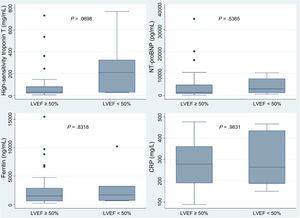
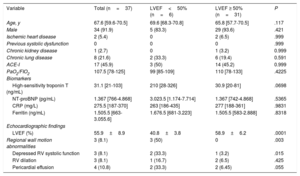
During the recruitment period, 38 patients were identified with confirmed COVID-19 and admitted to ICU due to respiratory distress syndrome. In 1 patient, ventricular function could not be assessed due to a poor acoustic window. The median age was 67.6 years and most of the patients were men (91.9%) (table 1). None of the patients had a history of heart failure or known LV systolic dysfunction. The median PaO2/FiO2 ratio was 107.5. Six patients (16.2%) had an LVEF<50% (2 mild, 4 moderate depression). Half of these patients had regional wall motion abnormalities (all with coronary distribution; 2 were inferior and one was anterolateral) and the rest had diffuse hypocontractility. Three patients (8.1%) had reduced right ventricular systolic function (2 of them also had reduced LVEF). There was a high prevalence of pericardial effusion in these patients (33%). The peak high-sensitivity troponin T values were higher in patients with low LVEF (median 210 vs 30.9), although this difference was not statistically significant (P=.0698). In contrast, no differences were found in the peak values of N-terminal pro-brain natriuretic peptide, ferritin, or C-reactive protein (figure 1). Of the 37 patients included, 7 (18.9%) died during the median follow-up of 75 [71-82] days, none of whom had reduced ventricular function (mild or moderate depression in all cases). None of the variables analyzed (LVEF<50%, right ventricular dysfunction, pericardial effusion, or regional wall motion abnormalities) were associated with death or readmission during follow-up. All patients with ventricular dysfunction have been referred for a cardiology appointment in our hospital for further testing once routine tests and procedures can be carried out as normal.
Baseline characteristics of the 37 patients with COVID-19 admitted to the ICU due to acute respiratory distress syndrome
| Variable | Total (n=37) | LVEF<50% (n=6) | LVEF ≥ 50% (n=31) | P |
|---|---|---|---|---|
| Age, y | 67.6 [59.6-70.5] | 69.6 [68.3-70.8] | 65.8 [57.7-70.5] | .117 |
| Male | 34 (91.9) | 5 (83.3) | 29 (93.6) | .421 |
| Ischemic heart disease | 2 (5.4) | 0 | 2 (6.5) | .999 |
| Previous systolic dysfunction | 0 | 0 | 0 | .999 |
| Chronic kidney disease | 1 (2.7) | 0 | 1 (3.2) | 0.999 |
| Chronic lung disease | 8 (21.6) | 2 (33.3) | 6 (19.4) | 0.591 |
| ACE-I | 17 (45.9) | 3 (50) | 14 (45.2) | 0.999 |
| PaO2/FIO2 | 107.5 [78-125] | 99 [85-109] | 110 [78-133] | .4225 |
| Biomarkers | ||||
| High-sensitivity troponin T (ng/mL) | 31.1 [21-103] | 210 [28-326] | 30.9 [20-81] | .0698 |
| NT-proBNP (pg/mL) | 1.367 [766-4.868] | 3.023.5 [1.174-7.714] | 1.367 [742-4.868] | .5365 |
| CRP (mg/L) | 275.5 [187-370] | 263 [186-435] | 277 [188-361] | .9831 |
| Ferritin (ng/mL) | 1.505.5 [663-3.055.6] | 1.676.5 [681-3.223] | 1.505.5 [583-2.888] | .8318 |
| Echocardiographic findings | ||||
| LVEF (%) | 55.9±8.9 | 40.8±3.8 | 58.9±6.2 | .0001 |
| Regional wall motion abnormalities | 3 (8.1) | 3 (50) | 0 | .003 |
| Depressed RV systolic function | 3 (8.1) | 2 (33.3) | 1 (3.2) | .015 |
| RV dilation | 3 (8.1) | 1 (16.7) | 2 (6.5) | .425 |
| Pericardial effusion | 4 (10.8) | 2 (33.3) | 2 (6.45) | .055 |
ACE-I, angiotensin-converting enzyme inhibitors; CRP, C-reactive protein; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; PaO2/FIO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; RV, right ventricle.
Values are expressed as No. (%), mean ± SD or median [interquartile range].
This is the first prospective study in our setting to assess acute myocardial injury in critical patients with severe acute respiratory distress syndrome due to COVID-19 based on biomarkers and echocardiographic findings. The prevalence of reduced LVEF in our series was higher than expected (16.2%) and higher than in previously published retrospective studies. In a recent study of 419 patients with COVID-19, of whom 36 required ICU admission, 11% of this ICU group had LV dysfunction defined as an LVEF<55%.4 Deng et al.5 described a prevalence of LV dysfunction (LVEF<50%) of 7.5% in a cohort of 67 patients admitted with severe disease. Of note, in our cohort, these patients had higher levels of high-sensitivity troponin T and a higher prevalence of pericardial effusion (33.3%), although this was not associated with increased mortality or readmission, perhaps because the reduction was mild to moderate in all cases.
In our unselected cohort of critical patients with COVID-19 admitted to ICU, LV dysfunction determined on handheld ultrasound was not associated with higher mortality. These results support the recommendations of the Spanish Society of Cardiac Imaging that, given the risk of echocardiography, its use should be limited, even in critical patients, to only certain subgroups of patients such as those with heart failure, arrhythmias, electrocardiographic changes, or cardiomegaly.
The authors thank Tomás Benito-González for his help in preparing this article.