Although coronavirus disease 2019 (COVID-19) is a systemic viral infection leading to severe acute respiratory syndromes, an increasing number of reports suggest that myocardial involvement is common and is associated with higher mortality.1 It has been observed that left (LV) and right ventricular (RV) abnormalities may not be uncommon, raising concern for systemic inflammation.2 However, there are few data on the performance of transthoracic echocardiography (TTE) to understand whether myocardial injury is a bystander phenomenon or a contributor to severe damage. Thus, this study aimed to define abnormalities on advanced TTE analysis in acute COVID-19 infection and to determine their implications in management and prognosis.
We performed a prospective cohort study including 200 patients admitted with COVID-19 and undergoing a TTE at the discretion of the clinician between March 1 and May 25, 2020. Due to the lack of familiarity with COVID-19, imaging was limited to patients who were expected to derive a benefit from its findings.3 Exclusion criteria were as follows: absence of confirmed SARS-CoV-2, age<18 years, handheld echocardiograms, and lack of quality. Each patient's chart was reviewed following TTE assessment to evaluate changes in management: treatment changes (antibiotics, diuretics, anticoagulation), hemodynamic support titration, facilitating decisions regarding patient care level, and no changes. Echocardiographic assessment, 2D-strain imaging, and myocardial work analysis was performed. Approval for the study was obtained from the center's Institutional Review Board. All patients included in the study signed the consent form prior to inclusion.
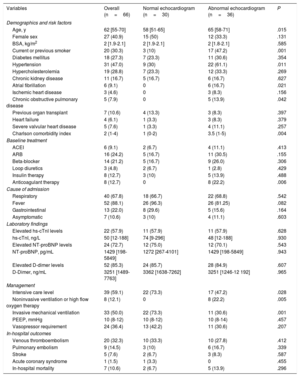
Sixty-six studies were included in the final analysis after exclusion of 134 patients (handheld echocardiograms, not following quality protocols). No differences regarding demographics and clinical characteristics were found between patients included and excluded in the analysis (P>.05). The median age was 62 years [IQR, 55-70] and 59.1% of patients were males (Table 1). Median time between hospital admission and TTE was 14 days [IQR, 6–22]. Indication for TTE was: 50% systemic conditions concern (endocarditis, pulmonary embolism), 30.3% hemodynamic assessment (shock, heart failure), 19.7% cardiac conditions (elevated biomarkers, chest pain). Patients with an abnormal TTE were older and presented more cardiovascular risk factors compared with patients with a normal TTE. Overall, 36 patients (54.5%) had an abnormal TTE study (Table 2). The most frequent abnormality was diastolic dysfunction (defined according to the 2016 ASE/EACVI guidelines) (33.3%), followed by RV dysfunction (12.1%), LV dysfunction (6.1%), and severe valvular heart disease or endocarditis (3%). 2-dimensional strain imaging and myocardial work analysis were performed only in 33 and 16 patients, respectively, due to the required high-resolution image quality. LV global longitudinal strain (GLS) was reduced in 48.5% of the patients and myocardial work performance were all reduced in patients with an abnormal TTE, although differences were not significant. The RV was dysfunctional in 12.1% and RV strain was reduced in 17.7% of the patients. There were no significant differences between a normal or an abnormal TTE study and the presence of elevated high-sensitivity troponin I (hs-cTnI), N-terminal pro-B-type natriuretic peptide (NT-proBNP), or D-dimer levels (P>.3 for all parameters). LV performance assessed by GLS showed a significant association with hs-cTnI (r=-0.556, P=.039), as well as global myocardial work index (GWI) (r=−0.900, P=.037). An abnormal TTE was one of the steps that impacted the clinical decision-making process in 60 patients: 28 treatment changes, 22 discharges from intensive care, and 10 titrations of hemodynamic support. The median length of hospital stay was 34 (interquartile range [IQR], 16-49) days, and in-hospital death did not significantly differ between a normal or abnormal TTE result.
Demographic and clinical characteristics of patients with and without a normal echocardiogram.
| Variables | Overall (n=66) | Normal echocardiogram (n=30) | Abnormal echocardiogram (n=36) | P |
|---|---|---|---|---|
| Demographics and risk factors | ||||
| Age, y | 62 [55-70] | 58 [51-65] | 65 [58-71] | .015 |
| Female sex | 27 (40.9) | 15 (50) | 12 (33.3) | .131 |
| BSA, kg/m2 | 2 [1.9-2.1] | 2 [1.9-2.1] | 2 [1.8-2.1] | .585 |
| Current or previous smoker | 20 (30.3) | 3 (10) | 17 (47.2) | .001 |
| Diabetes mellitus | 18 (27.3) | 7 (23.3) | 11 (30.6) | .354 |
| Hypertension | 31 (47.0) | 9 (30) | 22 (61.1) | .011 |
| Hypercholesterolemia | 19 (28.8) | 7 (23.3) | 12 (33.3) | .269 |
| Chronic kidney disease | 11 (16.7) | 5 (16.7) | 6 (16.7) | .627 |
| Atrial fibrillation | 6 (9.1) | 0 | 6 (16.7) | .021 |
| Ischemic heart disease | 3 (4.6) | 0 | 3 (8.3) | .156 |
| Chronic obstructive pulmonary disease | 5 (7.9) | 0 | 5 (13.9) | .042 |
| Previous organ transplant | 7 (10.6) | 4 (13.3) | 3 (8.3) | .397 |
| Heart failure | 4 (6.1) | 1 (3.3) | 3 (8.3) | .379 |
| Severe valvular heart disease | 5 (7.6) | 1 (3.3) | 4 (11.1) | .257 |
| Charlson comorbidity index | 2 (1-4) | 1 (0-2) | 3.5 (1-5) | .004 |
| Baseline treatment | ||||
| ACEI | 6 (9.1) | 2 (6.7) | 4 (11.1) | .413 |
| ARB | 16 (24.2) | 5 (16.7) | 11 (30.5) | .155 |
| Beta-blocker | 14 (21.2) | 5 (16.7) | 9 (26.0) | .306 |
| Loop diuretics | 3 (4.8) | 2 (6.7) | 1 (2.8) | .429 |
| Insulin therapy | 8 (12.7) | 3 (10) | 5 (13.9) | .488 |
| Anticoagulant therapy | 8 (12.7) | 0 | 8 (22.2) | .006 |
| Cause of admission | ||||
| Respiratory | 40 (67.8) | 18 (66.7) | 22 (68.8) | .542 |
| Fever | 52 (88.1) | 26 (96.3) | 26 (81.25) | .082 |
| Gastrointestinal | 13 (22.0) | 8 (29.6) | 5 (15.6) | .164 |
| Asymptomatic | 7 (10.6) | 3 (10) | 4 (11.1) | .603 |
| Laboratory findings | ||||
| Elevated hs-cTnI levels | 22 (57.9) | 11 (57.9) | 11 (57.9) | .628 |
| hs-cTnI, ng/L | 50 [12-188] | 74 [9-296] | 48 [12-188] | .930 |
| Elevated NT-proBNP levels | 24 (72.7) | 12 (75.0) | 12 (70.1) | .543 |
| NT-proBNP, pg/mL | 1429 [198-5849] | 1272 [267-4101] | 1429 [198-5849] | .943 |
| Elevated D-dimer levels | 52 (85.3) | 24 (85.7) | 28 (84.9) | .607 |
| D-Dimer, ng/mL | 3251 [1489-7763] | 3362 [1638-7262] | 3251 [1246-12 192] | .965 |
| Management | ||||
| Intensive care level | 39 (59.1) | 22 (73.3) | 17 (47.2) | .028 |
| Noninvasive ventilation or high flow oxygen therapy | 8 (12.1) | 0 | 8 (22.2) | .005 |
| Invasive mechanical ventilation | 33 (50.0) | 22 (73.3) | 11 (30.6) | .001 |
| PEEP, mmHg | 10 (8-12) | 10 (8-12) | 10 (8-14) | .457 |
| Vasopressor requirement | 24 (36.4) | 13 (42.2) | 11 (30.6) | .207 |
| In-hospital outcomes | ||||
| Venous thromboembolism | 20 (32.3) | 10 (33.3) | 10 (27.8) | .412 |
| Pulmonary embolism | 9 (14.5) | 3 (10) | 6 (16.7) | .339 |
| Stroke | 5 (7.6) | 2 (6.7) | 3 (8.3) | .587 |
| Acute coronary syndrome | 1 (1.5) | 1 (3.3) | 0 | .455 |
| In-hospital mortality | 7 (10.6) | 2 (6.7) | 5 (13.9) | .296 |
ACEI, angiotensin-converting-enzyme inhibitors; ARB, angiotensin II receptor blockers; BSA, body surface area; hs-cTnI; high-sensitivity cardiac-specific troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PEEP, positive end-expiratory pressure.
Categorical values are expressed as No. (%) and continuous values as median [interquartile range]
Echocardiographic findings in patients with a normal and abnormal echocardiographic study and COVID-19.
| Variables | Normal echocardiogram (n=30) | Abnormal echocardiogram (n=36) | P |
|---|---|---|---|
| Left ventricle | |||
| LV dysfunction | 0 | 7 (19.4) | .011 |
| LV end-diastolic dimension, mm | 44 (40-50) | 45 (41-50) | .775 |
| LV end-systolic dimension, mm | 27 (23-31) | 29 (24-33) | .314 |
| LV ejection fraction 4Ch, % | 63 (60-66) | 57 (52-65) | .032 |
| LV GLS, % | −18.2 [−14.2-22.7] | −17.1 [−12.3-22.2] | .649 |
| Reduced LV GLS | 7 (50) | 9 (47.4) | .580 |
| Regional motion abnormalities | 0 | 5 (13.9) | .042 |
| Right ventricle | |||
| RV dysfunction | 0 | 8 (22.2) | .006 |
| TAPSE, mm | 22 [20-24] | 19 (18-22) | .031 |
| Reduced TAPSE | 0 | 8 (22.2) | .021 |
| S’ wave, cm/s | 14.7 [12-17] | 13.8 [12-15.5] | .452 |
| RV fractional area change % | 50 [43-59] | 45 [41-54] | .157 |
| Reduced RV fractional area change | 0 | 5 (19.2) | .158 |
| RVFWLS % | −25 [−23.5-27.2] | −23 [-21-29] | .955 |
| Reduced RVFWLS | 0 | 3 (23.1) | .421 |
| Myocardial work (available for 16 patients) | |||
| Reduced GWI | 0 | 3 (30) | .250 |
| GWI, mmHg% | 2010 [1780-2138] | 1908 [1474-2362] | |
| Reduced GCW | 1 (16.7) | 3 (30) | .999 |
| GCW, mmHg% | 2211 [1966-2374] | 2185 [1513-2650] | |
| Elevated GWW | 0 | 4 (40) | .234 |
| GWW, mmHg% | 68 [49-73] | 124 [80-142] | |
| Reduced GWE | 0 | 4 (40) | .234 |
| GWE, % | 96 [95-97] | 93 [89-96] | |
| Diastolic function | |||
| LV diastolic dysfunction | 0 (0) | 28 (77.8) | .001 |
| Types of diastolic dysfunction | .001 | ||
| Diastolic dysfunction type I | 0 (0) | 23 (63.9) | |
| Diastolic dysfunction type II | 0 (0) | 2 (5.6) | |
| Diastolic dysfunction type III | 0 (0) | 3 (8.3) | |
| E/A ratio | 1.1 [0.9-1.2] | 0.7 [0.6-1.0] | .002 |
| Mitral valve deceleration time, ms | 219 [202-233] | 207 [275-250] | .414 |
| E/e’ ratio | 9 [7-10] | 9 [7-11] | .947 |
| Elevated E/e’ ratio | 0 | 3 (15) | .244 |
| Mitral regurgitation | |||
| None | 17 (73.9) | 22 (61.1) | .305 |
| Mild-moderate | 6 (26.1) | 13 (36.1) | .402 |
| Severe | 0 | 1 (2.8) | .610 |
| Tricuspid regurgitation | |||
| None | 10 (50) | 19 (52.8) | .531 |
| Mild-moderate | 10 (50) | 15 (41.7) | .586 |
| Severe | 0 | 2 (5.6) | .532 |
| Severe valvular heart disease | 0 | 5 (13.9) | .034 |
| Pulmonary hypertension (RVSP ≥ 35mm Hg) | 2 (6.8) | 9 (25.0) | .047 |
FAC, fraction area change; GCW, global constructive work; GLS, global longitudinal strain; GWE, global work efficiency; GWI, global work index; GWW, global wasted work; LV, left ventricle; RV, right ventricle; RVFWLS, right ventricle free wall longitudinal strain; RVSP, right ventricle systolic pressure.
Data are expressed as no. (%) or median [interquartile range].
To our knowledge, this is the first prospective report on a cohort of selected patients with COVID-19 infection admitted to a tertiary referral center undergoing TTE at the physician's discretion. The main findings are: a) more than half of the patients with COVID-19 had an abnormal TTE study and the most prevalent abnormality was diastolic dysfunction, with only less than 12% of the patients showing RV or LV dysfunction; b) patients with an abnormal TTE study were older and had more cardiovascular risk factors than patients with a normal TTE; c) there were no significant differences between TTE result and cardiac biomarkers; d) the most common indications were concerns about a systemic condition and the TTE result directly modified management in most cases, being one of the analytic steps in the treatment decision-making process.
Recent studies showing troponins to be associated with higher C-reactive protein, cytokines and NT-proBNP levels in SARS-CoV-2 infection have suggested a link between myocardial injury, inflammation, and ventricular dysfunction1; however, these studied lack imaging findings. In our study, despite biological cardiac injury, LV systolic dysfunction and wall motion abnormalities were uncommon, suggesting it may be related to the inflammatory syndrome. LV GLS has been described to be reduced in 52% to 70% of COVID-19 patients, emerging as a strong predictor of mortality4 and, in our data, myocardial work analysis was also significantly associated with hs-cTnI levels. Therefore, the most prevalent findings were subclinical changes, reinforcing evidence from other cohort studies, that cardiac involvement is high but mainly subclinical4,5 (reduced GLS and persistent myocardial inflammation on cardiovascular magnetic resonance). In our cohort, strain and myocardial work analysis were not considered as surrogate markers of LV dysfunction in COVID-19 patients with a normal echocardiogram and did not influence the decision-making process. It remains unknown whether clinical decisions based on these parameters result in a better outcome. Further multimodality imaging and large-scale biomarker studies are necessary to understand the pathophysiology. In previous reports, a major cardiovascular event was the main factors indicating TTE2; however, in our study, the most frequent indicator was a systemic condition, because myocardial injury was carefully interpreted with integration of symptoms, electrocardiographic changes, and the likelihood of coronary disease. Based on our results and in agreement with previous publications,6 an echocardiographic study should be limited to patients with a primary concern about a systemic condition, to rule out long-term intensive care unit complications, or to evaluate causes of hemodynamic instability and facilitate the decision-making process regarding patient care level and de-escalation of medical treatments.
This study has the limitations of selection bias, as echocardiography and biomarker testing were left to the physician's decision. Second, the single site and small sample size may have led to type II errors. However, the study was performed in a tertiary center representative of a large suburban area admitting 2025 patients with COVID-19 during the first wave of the pandemic. Third, it is unknown whether imaging abnormalities (diastolic dysfunction) were previously present and were thus unrelated to the infection. Finally, our results should be interpreted in light of the low mortality of our population and the absence of a short-term impact does not allow conclusions to be drawn on the absence of long-term consequences.
In conclusion, severe echocardiographic abnormalities are uncommon in hospitalized patients with COVID-19 infection, who show mostly subclinical myocardial changes. However, in these patients echocardiographic study is useful to guide the treatment and clinical decision-making process.
FUNDINGThere are no funding sources for this article for any author.
AUTHORS’ CONTRIBUTIONSAll authors had access to the data and participated in the preparation of this manuscript. All authors have contributed to the conceptualization of the study, data curation, formal analysis, investigation, methodology, validation, writing, and reviewing.
CONFLICTS OF INTERESTNone.