Advanced heart failure (HF) leads to high hospitalization and mortality rates. The LION-HEART study was a randomized, placebo-controlled clinical trial that evaluated the safety and efficacy of intravenous administration of intermittent doses of levosimendan in outpatients with advanced HF. The aim of the present study was to perform a cost analysis to determine whether the lower rate of hospitalizations for HF, observed in patients treated with levosimendan in the LION-HEART study, can generate savings for the Spanish national health system compared with the option of not treating patients with advanced HF.
MethodsAn economic model was used that included IC hospitalization rates from the LION-HEART study, the costs of hospitalization due to HF and those of the acquisition and intravenous administration of levosimendan. The time horizon of the analysis was 12 months. Two analyses were carried out, one deterministic and the other probabilistic (second-order Monte Carlo simulation).
ResultsIn the deterministic analysis, the total saving for each patient treated with levosimendan would amount to−€698.48. In the probabilistic analysis, the saving per patient treated with levosimendan would be−€849.94 (95%CI, €133.12 to−€2,255.31). The probability of savings with levosimendan compared with the no treatment option would be 94.8%.
ConclusionsIntermittent ambulatory treatment with levosimendan can generate savings for the Spanish national health system compared with the option of not treating patients with advanced HF.
Keywords
Heart failure (HF) is a progressive syndrome characterized by worsening symptoms, acute decompensation prompting unscheduled hospitalizations, the development of complications (eg, atrial arrhythmias), and a reduction in life expectancy.1 Over the last few years, substantial advances have been made in treatments for patients with HF due to systolic dysfunction. These include angiotensin receptor-neprilysin inhibitors, which improve prognosis; intravenous iron therapy, which enhances functional capacity and quality of life; and sodium-glucose cotransporter type 2 inhibitors, especially empagliflozin, which hold huge promise for reducing HF hospitalizations.2 Advanced HF can be defined as chronic HF that is not necessarily irreversible, meeting the following criteria: 1) refractory symptoms of New York Heart Association (NYHA) functional class III-IV together with elevated natriuretic peptides; 2) severe ventricular dysfunction, defined by a left ventricular ejection fraction <30%; 3) recurrent hospitalizations, unscheduled consultations, or malignant ventricular arrhythmias; and 4) limited exercise capacity of cardiovascular origin (6min walking test <300 m, peak oxygen consumption <14mL/kg/min or <50% of the predicted value for the patient's age).3 Medical treatment and device use, applying the recommendations in clinical practice guidelines, have led to improvements in HF-related morbidity and mortality.4
Hospitalization represents the greatest cost burden in HF and is much higher than the cost of drugs. Hence, strategies are needed to help reduce HF-related hospitalization episodes.5 In addition, there is a correlation between the HF treatment expenditure in specific patients and the severity of their disease. This relationship is not linear, as costs grow almost exponentially with increases in the patients’ NYHA functional class.6
The use of intermittent or continuous inotropic drug doses is considered a potentially effective approach to improve the clinical status of patients with advanced HF, especially those experiencing frequent hospitalizations.7
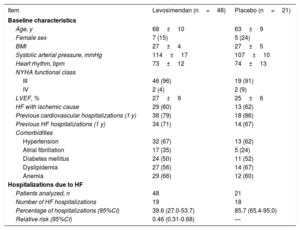
LION-HEART is a randomized, double-blind, parallel-group, multicenter clinical trial, whose objective was to evaluate the safety and efficacy of intermittent levosimendan (Simdax) administration compared with placebo (2:1) in patients with advanced HF.8 According to the LION-HEART results, the hospitalization rate in HF patients receiving levosimendan (39.6%) was significantly lower than that of the group receiving a placebo (85.7%)8 (table 1).
LION-HEART clinical trial. Main baseline characteristics of the patients and HF hospitalizations8
| Item | Levosimendan (n=48) | Placebo (n=21) |
|---|---|---|
| Baseline characteristics | ||
| Age, y | 68±10 | 63±9 |
| Female sex | 7 (15) | 5 (24) |
| BMI | 27±4 | 27±5 |
| Systolic arterial pressure, mmHg | 114±17 | 107±10 |
| Heart rhythm, bpm | 73±12 | 74±13 |
| NYHA functional class | ||
| III | 46 (96) | 19 (91) |
| IV | 2 (4) | 2 (9) |
| LVEF, % | 27±9 | 25±6 |
| HF with ischemic cause | 29 (60) | 13 (62) |
| Previous cardiovascular hospitalizations (1 y) | 38 (79) | 18 (86) |
| Previous HF hospitalizations (1 y) | 34 (71) | 14 (67) |
| Comorbidities | ||
| Hypertension | 32 (67) | 13 (62) |
| Atrial fibrillation | 17 (35) | 5 (24) |
| Diabetes mellitus | 24 (50) | 11 (52) |
| Dyslipidemia | 27 (56) | 14 (67) |
| Anemia | 29 (66) | 12 (60) |
| Hospitalizations due to HF | ||
| Patients analyzed, n | 48 | 21 |
| Number of HF hospitalizations | 19 | 18 |
| Percentage of hospitalizations (95%CI) | 39.6 (27.0-53.7) | 85.7 (65.4-95.0) |
| Relative risk (95%CI) | 0.46 (0.31-0.68) | — |
95%CI, 95% confidence interval; BMI, body mass index; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Values are expressed as No. (%) or the mean±standard deviation.
The aim of the present study was to perform a cost analysis to determine whether the lower HF hospitalization rate observed in advanced HF patients treated with intermittent levosimendan would lead to cost savings for the Spanish Health System compared with the option of not treating patients with this drug.
METHODSThe baseline characteristics of the patients included in the model are those of the participants in the LION-HEART8 clinical trial (table 1).
The cost analysis was modeled using Microsoft Excel and included the expenditure associated with the following items: levosimendan (Simdax) acquisition, hospitalization of patients with advanced HF, and levosimendan administration (cost of the time spent by the hospital nurse for intravenous drug delivery). The expenditure for adverse events (AEs) was not included in the base case analysis, as LION-HEART found no significant differences between the levosimendan and placebo groups regarding treatment-related severe or overall AEs. Severe treatment-related AEs were observed in 3 of the 48 patients treated with levosimendan (6.2%) and in 2 of the 21 patients receiving a placebo (9.1%) (P=0.646). As to overall treatment-related AEs, there were 5 events in the levosimendan group (10.4%) and 2 in controls (9.1%) (p=0.999).8 Nonetheless, a sensitivity analysis was carried out including all severe treatment-related AEs.
In the LION-HEART study, the following differences in the use of health resources were observed between patients receiving levosimendan and those with placebo (designated no treatment in the model): 1) levosimendan treatment would lead to cost savings derived from the lower hospitalization rate in treated vs untreated HF patients: 39.6% (19 hospitalizations in 48 patients) and 85.7% (18 hospitalizations in 21 patients), respectively8 (table 1); and 2) levosimendan treatment would involve 2 additional costs, the cost of acquiring levosimendan9 and the cost of administering the drug by intravenous perfusion in the day hospital.10
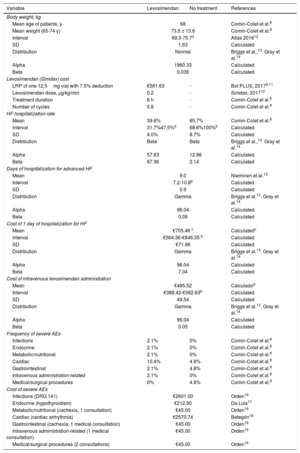
All the direct health costs per unit (analysis performed from the perspective of the Spanish National Health System) were obtained from Spanish sources. The retail price of levosimendan provided by the laboratory (€628.79 for a 12.5-mg vial; €50.30 per mg) was obtained from the Bot PLUS database (table 2). A 7.5% deduction was applied to the price of levosimendan, in accordance with the Royal Decree-Law 8/2010.11
Variables included in the cost analysis model
| Variable | Levosimendan | No treatment | References |
|---|---|---|---|
| Body weight, kg | |||
| Mean age of patients, y | 68 | Comín-Colet et al.8 | |
| Mean weight (65-74 y) | 73.5 ± 13.6 | Comín-Colet et al.8 | |
| Interval | 69.3-75.7a | Atlas 201612 | |
| SD | 1.63 | Calculated | |
| Distribution | Normal | Briggs et al.,13. Gray et al.14 | |
| Alpha | 1960.33 | Calculated | |
| Beta | 0.036 | Calculated | |
| Levosimendan (Simdax) cost | |||
| LRP of one 12.5mg vial with 7.5% deduction | €581.63 | - | Bot PLUS, 20179.11 |
| Levosimendan dose, μg/kg/min | 0.2 | - | Simdax, 201710 |
| Treatment duration | 6 h | - | Comín-Colet et al.8 |
| Number of cycles | 5.8 | - | Comín-Colet et al.8 |
| HF hospitalization rate | |||
| Mean | 39.6% | 85.7% | Comín-Colet et al.8 |
| Interval | 31.7%47.5%b | 68.6%100%b | Calculated |
| SD | 4.0% | 8.7% | Calculated |
| Distribution | Beta | Beta | Briggs et al.,13. Gray et al.14 |
| Alpha | 57.63 | 12.86 | Calculated |
| Beta | 87.96 | 2.14 | Calculated |
| Days of hospitalization for advanced HF | |||
| Mean | 9.0 | Nieminen et al.15 | |
| Interval | 7.2-10.8b | Calculated | |
| SD | 0.9 | Calculated | |
| Distribution | Gamma | Briggs et al.13. Gray et al.14 | |
| Alpha | 96.04 | Calculated | |
| Beta | 0.09 | Calculated | |
| Cost of 1 day of hospitalization for HF | |||
| Mean | €705.46 c | Calculatedc | |
| Interval | €564.36-€846.55 b | Calculated | |
| SD | €71.98 | Calculated | |
| Distribution | Gamma | Briggs et al.13. Gray et al.14 | |
| Alpha | 96.04 | Calculated | |
| Beta | 7.34 | Calculated | |
| Cost of intravenous levosimendan administration | |||
| Mean | €485.52 | Calculadod | |
| Interval | €388.42-€582.63b | Calculated | |
| SD | 49.54 | Calculated | |
| Distribution | Gamma | Briggs et al.13. Gray et al.14 | |
| Alpha | 96.04 | Calculated | |
| Beta | 5.05 | Calculated | |
| Frequency of severe AEs | |||
| Infections | 2.1% | 0% | Comín-Colet et al.8 |
| Endocrine | 2.1% | 0% | Comín-Colet et al.8 |
| Metabolic/nutritional | 2.1% | 0% | Comín-Colet et al.8 |
| Cardiac | 10.4% | 4.8% | Comín-Colet et al.8 |
| Gastrointestinal | 2.1% | 4.8% | Comín-Colet et al.8 |
| Intravenous administration-related | 2.1% | 0% | Comín-Colet et al.8 |
| Medical/surgical procedures | 0% | 4.8% | Comín-Colet et al.8 |
| Cost of severe AEs | |||
| Infections (DRG 141) | €2601.00 | Orden16 | |
| Endocrine (hypothyroidism) | €212.90 | De Luis17 | |
| Metabolic/nutritional (cachexia, 1 consultation) | €45.00 | Orden16 | |
| Cardiac (cardiac arrhythmia) | €2570.74 | Betegón18 | |
| Gastrointestinal (cachexia, 1 medical consultation) | €45.00 | Orden16 | |
| Intravenous administration-related (1 medical consultation) | €45.00 | Orden16 | |
| Medical/surgical procedures (2 consultations) | €45.00 | Orden16 | |
DRG, diagnosis-related group; EAs, adverse e vents; HF, heart failure; LRP, laboratory retail price; min, minute
Calculated using the cost of DRG 127 (HF) with an intravenous requirement, advanced HF (€6349.10) (2017 rates),19 updated to February, 2018 (National Statistics Institute, 2018).
The levosimendan dose administered in LION-HEART was 0.2μg/kg/min for 6hours, in a total of 5.8 cycles.8 The mean body weight of the patients in the model (73.5 ±13.6kg) was that of the LION-HEART patients,8 and the extreme body weights were those of the Spanish population aged 65 to 74 years, adjusted by sex, reported in the Atlas de la Sanidad en España (Atlas of Heath Care in Spain) of the Ministry of Health, Social Services, and Equality12 (table 2). Based on these assumptions, a patient would receive, in total, an average of 30.7mg of levosimendan.
The cost of HF hospitalization, obtained from the expense of NYHA1 class III and IV advanced HF from the publicly-funded health system of the Basque Country,19 was €6349.10 (table 2). The cost per day of hospitalization for patients with advanced HF was calculated from this price per unit and assuming 9 days of hospitalization, in accordance with the mean length of hospital stay reported in the EuroHeart Failure Survey II15 registry, in which 65% of patients had reductions in the left ventricular ejection fraction.
The cost of intravenous levosimendan administration was estimated using the cost of the time spent by a hospital nurse to perform this task. The estimated length of intravenous administration was 6hours (360min), according to LION-HEART8 and a study by Bonios et al.20 The price of 1minute of nursing care for this purpose was €0.23, calculated from the yearly salary of a specialized hospital nurse in the Aragonese Health Service21 and taking into account that 5.8 cycles of the drug were given in the LION-HEART study.8
The cost of AEs occurring with and without levosimendan was calculated using the frequency data reported in LION-HEART.8 The unit costs of AEs were obtained from several Spanish sources16–18 (table 2).
The deterministic calculation of the cost of advanced HF per patient used the following model: 1) levosimendan cost was calculated by multiplying the price of one vial (€581.63) by the number of vials needed (3 vials for a total dose of 30.7mg per patient, with the dose calculated as follows: 0.2μg/kg/min×6hours of administration×5.8 cycles×73.45kg of body weight); 2) hospitalization cost was calculated by multiplying the probability of hospitalization with levosimendan (39.6%) and without levosimendan (85.7%) by the hospitalization cost (€6349.10); and 3) the cost of intravenous levosimendan administration by the hospital nurse was estimated by multiplying the 6hours (360min) of administration by the cost of 1minute of specialist nursing time (€0.23), by 5.8 cycles.
All costs were updated to the month of February, 2018, according to the Interannual Consumer Price Index set by the Spanish National Statistics Institute.22 The period of the analysis covered the 12 months of treatment and follow-up of patients in the LION-HEART study.8
Four analyses were conducted: 1) a deterministic base case analysis using the average probability values and unit costs (this is a fixed result); 2) a deterministic analysis including the cost of all treatment-related AEs; and 3) a probabilistic analysis (carried out using a second-order Monte Carlo simulation) with 1000 analyses for the following variables: body weight (normal distribution), hospitalization due to HF (beta distribution), number of hospitalizations per patient (gamma distribution), cost of hospitalization due to HF (gamma distribution), and day hospital cost (gamma distribution)13,14; and, lastly, 4) an additional probabilistic analysis calculating the cost of levosimendan treatment according to the price per milligram (€45.53) instead of the number of vials used. The variables included in the deterministic analyses (mean values) and probabilistic analyses (extreme values, standard deviations, alpha and beta statistics) are presented in table 2.
RESULTSDeterministic analysesThe cost calculations for levosimendan treatment are summarized in table 3. The total estimated cost of this treatment was €1744.89. The calculated cost of HF-related hospitalizations in patients receiving levosimendan compared with those with no treatment would be €2513.18 and €5442.08, respectively, yielding a cost saving of –€2928.90 with levosimendan use. The hospital nursing cost for intravenous levosimendan administration was €485.52. Hence, the total cost saving per patient treated with levosimendan would amount to €698.48 (table 3).
Results of the deterministic analysis. Costs per patient treated or not with levosimendan (€). Analysis by number of vials
| Item | Levosimendan | No treatment | Difference |
|---|---|---|---|
| Medication | 1744.89 | 0 | 1744.89 |
| Hospitalization for HF | 2513.18 | 5442.08 | –2928.90 |
| Intravenous drug administration | 485.52 | 0 | 485.52 |
| Total cost per patient | 4743.60 | 5442.08 | –698.48 |
HF, heart failure
When the cost of treatment-related AEs was included in the analysis, the saving per patient treated with levosimendan was €494.82.
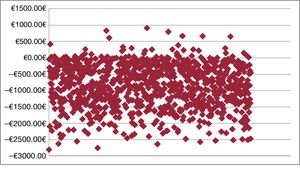
Probabilistic analysesThe probabilistic results obtained for each variable (medication, hospitalization due to HF, intravenous administration) are shown in table 4. The average total saving per patient treated with levosimendan would amount to –€849.94 (95% confidence interval [95% CI], €133.12 to –€2.255.31). That is, according to the model, the 95% CI of levosimendan was defined by an expenditure of €133.12 to a saving of –€2255.31. In the Monte Carlo simulation, there was a probability that levosimendan use would produce savings compared with the no treatment option in 94.8% of the simulations (figure 1); that is, in 5.2% of the results, there would be no savings with levosimendan.
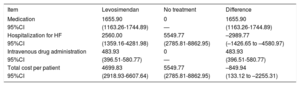
Results of the probabilistic analysis. Cost per patient treated or not with levosimendan (€). Analysis by number of vials
| Item | Levosimendan | No treatment | Difference |
|---|---|---|---|
| Medication | 1655.90 | 0 | 1655.90 |
| 95%CI | (1163.26-1744.89) | — | (1163.26-1744.89) |
| Hospitalization for HF | 2560.00 | 5549.77 | –2989.77 |
| 95%CI | (1359.16-4281.98) | (2785.81-8862.95) | (–1426.65 to –4580.97) |
| Intravenous drug administration | 483.93 | 0 | 483.93 |
| 95%CI | (396.51-580.77) | — | (396.51-580.77) |
| Total cost per patient | 4699.83 | 5549.77 | –849.94 |
| 95%CI | (2918.93-6607.64) | (2785.81-8862.95) | (133.12 to –2255.31) |
95%CI, 95% confidence interval; HF, heart failure
In the additional probabilistic analysis, calculation of the treatment cost per milligram of levosimendan yielded an average total savings per patient of –€1.123.22 (95% CI, –€87.99 to –€2130.47), with a 98.4% probability of savings compared with the no treatment option.
DISCUSSIONIn the LION-HEART8 trial, intermittent levosimendan administration in ambulatory patients with advanced systolic HF led to reductions in plasma concentrations of N-terminal pro-B-type natriuretic peptide, attenuated the worsening of health-related quality of life, and resulted in lower HF-related hospitalizations and mortality rates. Specifically, hospitalization was required in 39.6% of patients treated with intermittent levosimendan and 85.7% of those not receiving this drug.8
According to the cost analysis, the savings derived from the lower hospitalization rate associated with levosimendan use would compensate for the expenditure of the acquisition and administration (in the day hospital) of the drug. The cost saving obtained with levosimendan would amount to –€849.94 (95% CI, €133.12 to –€2.255.31), with a 94.8% probability that savings would be generated compared with the no treatment option. In the cost analysis performed per milligram of drug, the probability increased to 98.4%.
One important indirect consequence of reducing HF hospitalizations in a universal, publically-funded health system such as that of Spain is that “potential hospital stays” become available, and these can be used to resolve other conditions. As a larger number of patients can be accommodated, this would contribute to reducing hospital waiting lists.23
To our knowledge, there are no other national or international studies investigating the specific aims of the present analysis. Nonetheless, there are some cost analyses related to intermittent use of other inotropic drugs, such as dobutamine and phosphodiesterase 3 inhibitors. Marius-Nunez et al.24 evaluated the use of dobutamine or a phosphodiesterase 3 inhibitor (milrinone) in previously hospitalized HF patients, using a 4-hour ambulatory administration regimen. Hospital admissions and emergency room visits for HF were significantly reduced. In general, there was an 86% decrease in hospital expenditure (P <.001).24 In another study performed in 41 patients in NYHA functional class III-IV, intermittent use of a phosphodiesterase 3 inhibitor (amrinone) every 2 to 6 weeks based on the patients’ symptoms, resulted in a 56% reduction in HF hospitalizations (P <0.05), which was considered to demonstrate the cost-effectiveness of this intervention.25
The potential limitations and strengths of the present study should be taken into consideration when evaluating the results. Regarding its limitations, the study used a theoretical model which, by definition, is a simplified simulation of reality. Furthermore, the values for some variables had to be estimated because they were not provided in the LION-HEART8 clinical trial. This was the case of the time spent by the hospital nurse for levosimendan administration, which was estimated to be equivalent to the time needed for intravenous perfusion.8,15 The average length of the hospital stay for a patient with advanced HF, also estimated for this study, was considered to be similar to that of a patient with decompensated HF. Decompensation is a common reason for hospitalization in patients with advanced HF,1 and the average length associated with the diagnosis-related group 127 might underestimate that of an advanced HF patient.
Two assumptions were considered in the cost estimate of levosimendan treatment: 1) that the amount remaining in a vial would not be used to treat subsequent patients (conservative assumption, and 2) that the vials would be fully used. Both these options are common in clinical practice. From the microbiological viewpoint, the medication should be used immediately, and if this is not the case, the storage time during use and the conditions before use are the responsibility of the user. Usually, the medication is stored for less than 24hours at 2 to 8°C, unless dilution is carried out in controlled and validated aseptic conditions. The storage and use time after dilution should never exceed 24hours.10
The external validity of a cost analysis depends on the external validity of the clinical study used as the basis. In this regard, the small sample size in the LION-HEART trial should be taken into account, although it sufficed to detect statistically significant differences in the hospitalization rates.8
The present analysis included direct health care costs, which had different degrees of uncertainty regarding their true impact on hospital expenditure. The outlay for hospitalization and for treatment administration by a nurse are fixed costs that would be produced in all cases, whereas the price of the medication is variable.
This study also has several strengths. The hospitalization rates with and without levosimendan administration were obtained from a randomized, double-blind clinical trial,8 and the probabilistic analysis confirmed the robustness of the main finding: the 94.8% probability that cost savings would be generated with levosimendan use relative to the no treatment option. The estimated intravenous administration cost was based on the consideration that the hospital nurse would devote 6 full hours to each cycle. This is a conservative assumption, as a nurse attends several patients simultaneously in clinical practice. Furthermore, the analysis did not include health care-unrelated direct costs or indirect costs associated with hospitalization of HF patients. Specifically, an estimated 59% to 69% of the total expenditure for the disease is attributable to these sources.5,26 It is likely that the savings obtained with levosimendan use would have been even higher if these factors had been taken into account.
Finally, a new study named LeoDOR (levosimendan infusion for patients with advanced chronic heart failure), which is now in the recruitment phase, will investigate intermittent levosimendan administration using 2 treatment schemes. The primary outcome measure in this study has 3 hierarchically established components: mortality, implantation of a mechanical ventricular assist device/cardiac transplantation, and changes in N-terminal pro-B-type natriuretic peptide.24 The participants will be patients previously hospitalized for decompensated HF. Therefore, levosimendan treatment will be particularly focused on the vulnerable phase following an important event such as HF hospitalization.27
CONCLUSIONSAccording to the cost analysis applied to the results of the LION-HEART trial, intermittent outpatient treatment with levosimendan in advanced HF patients can generate savings for the Spanish National Health System compared with the no treatment option, mainly due to a significant reduction in HF-related hospitalizations.
FUNDINGThis study was sponsored by Orion Pharma. The sponsorship source had no influence on the design of the cost analysis, the data analysis, or the publication process.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest, with the exception of the following: C. Rubio-Terrés, D. Rubio-Rodríguez, J. Comín-Colet, and N. Manito Lorite received payments from Orion Pharma in connection with the study. C. Campo Sien works at Orion Pharma.
- –
Hospitalization for HF represents the highest cost burden associated with this disease, and is much higher than the cost of pharmacological treatment.
- –
There is a substantial correlation between the cost of HF treatment and the severity of the patient's condition, which is highest in patients with advanced HF.
- –
Intermittent or continuous dosing of inotropic drugs such as levosimendan is considered a potentially effective approach for advanced HF patients, particularly those who experience frequent hospitalizations.
- –
This is the first national or international study analyzing the cost impact on the National Health System of intermittent levosimendan outpatient treatment in patients with advanced HF.
- –
The savings for the Spanish National Health System generated by intermittent inotropic treatment with levosimendan mainly result from the significant reduction in HF hospitalizations.
- –
In this study, the fact that hospitalization rates with and without levosimendan were taken from a randomized double-blind clinical trial, lends robustness to the model applied and enhances the results.
José Manuel García Pinilla, Hospital Universitario Virgen de la Victoria, Malaga, Spain; Luis Almenar, Hospital Universitario y Politécnico La Fe, Valencia, Spain; María G. Crespo-Leiro, Complexo Hospitalario Universitario de A Coruña (CHUAC) e Instituto de Investigación Biomédica de A Coruña (INIBIC), Universidad de A Coruña (UDC), A Coruña, Spain; Alessandro Sionis, Hospital de la Santa Creu i Sant Pau, Instituto de Investigación Biomédica Sant Pau (IIB Sant Pau), Universitat Autònoma de Barcelona, Barcelona, Spain; Teresa Blasco, Hospital Universitario Miguel Servet, Zaragoza, Spain; Domingo Pascual-Figal, Hospital Universitario Virgen de la Arrixaca, Murcia, Spain; Francisco Gonzalez-Vilchez, Hospital Universitario Marqués de Valdecilla, Universidad de Cantabria, Santander, Spain; José Luis Lambert-Rodríguez, Hospital Universitario Central de Asturias, Oviedo, Spain; Maria Grau, Grupo de Investigación en Epidemiología y Genética Cardiovascular del Cardiovascular del IMIM (Instituto Hospital del Mar de Investigaciones Médicas) and Universidad de Barcelona, Barcelona, Spain.