Keywords
INTRODUCTION
Current guidelines on clinical practice recommend a routine invasive strategy (RIS) in patients with non-ST-segment elevation acute coronary syndrome (NSTEACS).1,2 The available evidence indicates a consistent reduction in minor adverse events when using an RIS to manage patients with NSTEACS.3,4 However, there is little information on reductions in death or infarction, and when this is available it is frequently contradictory.3,4 In contrast, and despite their limitations, observational studies, which commonly include patients with greater baseline risk, have shown that an RIS decreases the risk of major adverse events compared to a selective invasive or conservative revascularization strategy.5-9
Left ventricular systolic dysfunction (SD) is a known independent predictor of adverse events in patients with acute coronary syndrome10-12; however, the prognostic scores most commonly used in daily clinical practice do not include this among their components.13-15 Furthermore, the scientific evidence regarding this issue is mainly based on the results of contemporary clinical trials that either did not systematically assess systolic function,16-18 or, when they did,19 showed a marginal percentage of patients with SD. Recently, the results from the GRACE registry showed that revascularization was associated with a marked reduction in the risk of mortality after hospital discharge in the subgroup of patients with congestive heart failure (CHF).20 In the light of this, and due to the fact that conventional clinical examination has limited specificity and sensitivity to identify patients with ventricular dysfunction,21-22 we suggest that, in patients with NSTEACS, the identification of SD by routine echocardiographic examination would facilitate the early selection of the subgroup of patients with greater and more severe myocardial ischemia, and thus greater expected prognostic benefit from an RIS.
The aim of the present study was to establish whether the prognostic impact (long-term mortality or myocardial infarction) of coronary angiography and subsequent revascularization during hospitalization for NSTEACS differs according to the presence or absence of SD.
METHODS
Study Population
We analyzed a total of 1017 patients consecutively admitted to our hospital between January 2001 and May 2005 with a diagnosis of high-risk NSTEACS, defined by the presence of chest pain during the previous 24 hours and increased troponin I (TnI) levels or electrocardiographic evidence of ST segment depression. The patients who died during index hospitalization (n=45) were excluded from the present analysis, leaving 972 patients in the study group. The type of revascularization strategy followed was decided by the clinical cardiologist; however, as of 2002, due to the publication of new clinical guidelines,23 an RIS was recommended in these patients. The left ventricular ejection fraction (LVEF) was assessed by transthoracic echocardiography during initial admission. Coronary angiography, when performed, was conducted during index hospitalization at a mean of 96 (48) hours after admission. In 94% of catheterized patients, echocardiography preceded coronary angiography and in only 36 patients was coronary angiography performed after echocardiography.
For the main analysis, systolic function was considered preserved when LVEF was ≥50% and depressed if it was <50%. The LVEF was calculated using Simpson's method in patients with regional wall-motion abnormalities, and by using Teichholz's method in the other patients. All the patients were treated with aspirin and low-molecular-weight heparin. The use of glycoprotein IIb-Ila receptor inhibitors was limited to abciximab in the catheterization laboratory and its indication was decided by the interventional cardiologist. Any other pharmacological treatment was decided by the attending clinical cardiologist.
Definition of Adverse Events and Follow-up
The appearance during follow-up of all-cause mortality or myocardial infarction was the main endpoint in the present study. Acute myocardial infarction (AMI) was defined as follows: a) elevated cardiac enzyme markers (TnI or CK-MB) associated with typical chest pain or ST segment deviation; or b) increased CK-MB level 3 times its upper limit after percutaneous transluminal coronary angioplasty (PTCA) or more than 5 times after coronary revascularization surgery.24
Clinical follow-up was conducted during successive outpatient visits or, in their absence, by telephone contact (patients who, for whatever reason, could not attend programmed visits). The protocol followed in the present study was approved by the ethics committee of our hospital.
Statistical Analysis
The risk of death or AMI during follow-up for both categories (LVEF <50% and ≥50%) was stratified according to coronary angiography and revascularization procedures. The differences were estimated using Kaplan-Meier curves and were compared using the log-rank test.
Due to the observational character of the present study, and to the presence of baseline differences on coronary angiography (Table 1), we decided to create a propensity index to minimize this selection bias.25 Thus, by using a multivariate logistic regression model, we identified the variables associated with coronary angiography, which we consider to be the crucial and defining step for a patient to subsequently be revascularized. The propensity index was constructed by including all variables with a P≤.25 in the univariate analysis, as well as those that the literature has shown to be associated with invasive procedures regardless of the P value and the year of admission, since revascularization guidelines have changed over time. The final model used for constructing the propensity index included the following: age, sex, year of admission, smoking history, CHF history, stroke history, peripheral artery disease, chronic obstructive pulmonary disease, previous use of aspirin, ST segment deviation, serum creatinine level, presence of CHF, stress testing, and recurrent angina during hospitalization. The area under the receiver operating characteristic curve of the propensity index was 0.89, indicating excellent discrimination. Cox proportional hazard analysis was used to determine the risk of a combined episode for long-term mortality and myocardial infarction. The final multivariate model was adjusted by the propensity index (individual probability of being catheterized), dividing into quintiles the TIMI risk score,16 comorbidity estimated by the Charlson index,26 Killip class >I at admission or during hospitalization, and elevated serum creatinine levels. The proportional hazards assumption was assessed by analysis of Schoenfeld residuals. The estimated coefficients were expressed as hazard ratio (HR) with their respective 95% confidence intervals (95% CI). The final discriminatory capacity of the prognostic multivariate models was determined using Harrell's C statistic. In all cases, a P<.05 was used as the cutoff for statistical significance. The STATA 9.2 software package was used for the statistical analysis.
RESULTS
Baseline Characteristics of the Population
The mean age of our sample was 68.7 (12.4) years, 625 (64.3%) were men, 343 (35.3%) had diabetes mellitus, 825 (84.9%) presented elevated troponin I levels, 459 (47.2%) had ST segment depression, 152 (15.6%) were in Killip class >I, and 227 (23.4%) had an LVEF <50%. The proportion of catheterized and revascularized patients during the index event was 62.4% and 38.7%, respectively. The most frequent revascularization modality used was percutaneous intervention (75%) and a stent was used in 94.7% of these procedures. In general, the catheterized and revascularized patients presented a better baseline risk profile (Tables 1 and 2, respectively).
Baseline Characteristics Stratified According to Systolic Function
In total, 227 (23.4%) and 141 (14.5%) patients had an LVEF <50% and <45%, respectively. The patients with an LVEF <50% were older, presented greater creatinine levels at admission, had higher TIMI risk scores, and presented a greater percentage of diabetes mellitus, chronic kidney failure, stroke, previous aspirin use, and peripheral artery disease. Regarding treatment, the patients with SD were more frequently prescribed beta-blockers, and underwent coronary angiography and PTCA less frequently; in contrast, they underwent coronary revascularization surgery more frequently (Table 3). Finally, no differences were observed in the overall rates of revascularization according to the presence or absence of SD (Table 3).
Coronary Angiography and Long-term Adverse Events
During a median follow-up of 24 months (interquartile range, 6-42), 193 (19.9%) patients died, 176 (18.1%) presented an infarction and 303 (31.2%) reached the combined endpoint of death/AMI.
The proportion of death/AMI during follow-up was lower among the catheterized patients (21% vs 48.1%, P<.001) compared to noncatheterized patients. However, these prognostic differences were not homogeneous after stratifying the patients according to the presence or absence of SD. Thus, the cumulative probability of death, myocardial infarction and the combined endpoint of death and AMI, was considerably higher in the subgroup of noncatheterized patients with an LVEF <50% compared to the 3 remaining categories (noncatheterized patients, LVEF ≥50%; catheterized patients, LVEF <50%; and catheterized patients, LVEF ≥50%); these differences were already marked in the first days of follow-up (Figure 1).
Figure 1. Cumulative risk of death (A), AMI (B), and the combined endpoint of death/AMI (C) stratified according to coronary angiography and the presence of systolic dysfunction in patients with NSTEACS.
The differential prognostic effect attributable to performing coronary angiography according to the presence or absence of SD (P for interaction = .010) was confirmed by the multivariate analysis, after adjusting by the propensity index (individual probability of being catheterized), the TIMI risk score, accompanying comorbidity (Charlson index), elevated serum creatinine level, and Killip class >I at admission. Thus, those with an LVEF <50% showed greater prognostic benefit after catheterization (HR=0.47; 95% CI, 0.30-0.75; P=.001) than catheterized patients with an LVEF ≥50% (HR=0.90; 95% CI, 0.63-1.29; P=.567).
Coronary Angiography, Revascularization, and Long-term Prognosis
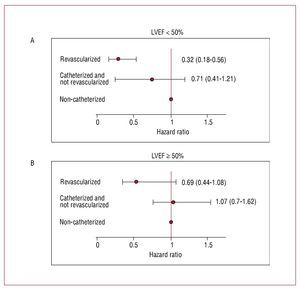
In a subsequent analysis, we investigated whether the prognostic differences observed between the catheterized patients in the 2 LVEF categories was due, at least partly, to the revascularization procedures. To this end, we divided our population into 3 groups: a) noncatheterized patients and, obviously, nonrevascularized patients (n=366, reference category); b) catheterized and nonrevascularized patients (n=230); and c) revascularized patients (n=376). After multivariate adjustment: a) the catheterized and nonrevascularized patients did not present better long-term prognosis, regardless of the presence or absence of SD (P for interaction = .201) as shown in Table 4 and Figure 2) the magnitude of the prognostic benefit attributable to revascularization was far greater in the patients with an LVEF <50% (P for interaction =.012). Thus, the reduction of risk attributable to coronary revascularization was 2 times greater in the patients with an LVEF <50% (HR=0.32; 95% CI, 0.18-0.56; P<.001) versus the patients with an LVEF ≥50%, where revascularization was marginally associated with long-term prognosis (HR=0.69; 95% CI, 0.44-1.08; P=.108) (Table 4). Harrell's C statistic for the final model was 0.734, indicating good discrimination.
Figure 2. Prognostic effect attributable to coronary angiography or revascularization procedures depending on the presence of systolic dysfunction in patients with NSTEACS. A: patients with systolic dysfunction. B: patients with preserved systolic function. Multivariate analysis adjusted by the propensity index (individual probability of being catheterized), for TIMI risk score, accompanying comorbidity (Charlson index), elevated serum creatinine levels and Killip class >I at admission.
The magnitude and direction of the results was substantially different in the 4 sensitivity analyses: a) adjusting the multivariate model for the pharmacological treatment groups at discharge; b) excluding those patients with Killip class >I; c) including only those with a history of AMI; and d) varying the LVEF cutoff point to define SD (LVEF<45%) (Table 4).
DISCUSSION
In the present study, we assessed a large consecutive cohort of patients with high-risk NSTEACS, and found that the greatest benefit attributable to routine coronary angiography was observed in the subgroup of patients with an LVEF <50%. As expected, this differential prognostic effect was confirmed in the revascularized patients. This differential prognostic effect was consistent despite adjusting by a propensity index (individual probability of undergoing coronary angiography), TIMI risk score, Charlson comorbidity index, Killip class >I and elevated serum creatinine levels. Furthermore, the same results were found after adjusting the model for the pharmacological treatment groups at discharge, excluding those with Killip class >I, varying the cutoff point defining SD, or even when only the subgroup of patients with a history of AMI was analyzed. In view of these results, we suggest that the routine and early assessment of LVEF in patients with NSTEACS would help to identify a population subgroup who would benefit from an RIS.
Systolic Dysfunction in Patients With Non-St-Segment Elevation Acute Coronary Syndrome. A Gap Between Observational Studies and Randomized Studies?
Unlike randomized studies, where there is scarce evidence in support of an RIS,3-4 the adoption of an RIS has been associated with a striking reduction in the risk of major adverse events in recently published observational studies.5-9 These studies included population subgroups classically under-represented in clinical trials (patients with heart failure, women, patients of advanced age, and patients with high comorbidity, among others). Despite the plethora of studies investigating the prognostic influence of an RIS on NSTEACS, the information available on this issue in patients with SD and NSTEACS is very limited.
Most clinical trials do not provide data related to LVEF,16-18 but when provided, the data are predominantly normal although the percentage of patients with SD is not specified.27-28 Only the ICTUS study reported that a marginal 14% of the study population presented an LVEF <35%.19 Despite the precautions taken when interpreting the analysis by population subgroups, most studies present their results grouped according to the classic variables (age, sex, diabetes, electrocardiographic alterations, and elevated biomarkers, among others); however, the absence of prognostic information related to the patients with SD is striking. Thus, only the old VANQWISH study reported that in the subgroup of patients with SD, no benefit attributable to an RIS was found, although neither the percentage of patients with SD nor the cutoff points used to define it were specified.27
The LVEF has not always been measured in observational studies,5,8,20 and thus it has not been routinely included as a prognostic variable. Furthermore, despite the fact that numerous studies investigating chronic ischemic heart disease have highlighted the benefit of revascularization in patients with SD,29 such studies are more than just scarce within the area of NSTEACS; in fact, there are none. In view of our results, we suggest that one of the possible differences that may explain the prognostic differences between randomized and observational studies resides, at least partly, in the fact that the proportion of patients with SD included in the observational studies was much higher than that in controlled studies.
Biological Plausibility of Our Findings
It is well known that LVEF is one of the more relevant independent predictors in patients with ACS.10-12 Within the area of chronic ischemic heart disease, a large body of evidence has indicated that ischemic patients with depressed LVEF and predicted recovery (using noninvasive myocardial viability testing) constitute a high-risk group in whom revascularization improves survival.29-30 However, in NSTEACS, the evidence in this regard is very limited, although certain observations suggest it. Plein et al reported that regional wall-motion abnormalities in patients with NSTEACS showed good diagnostic yield in predicting significant coronary artery disease on angiography.31 In a series of 601 patients assessed by magnetic resonance imaging for acute chest pain of possible coronary origin, Bodí et al demonstrated that the joint presence of inducible ischemia and regional wall-motion abnormalities identified the subgroup of patients who would obtain the greatest benefit from revascularization in terms of reducing the risk of cardiovascular episodes.32 Recently, in a series of 13 707 patients with ACS in the GRACE registry, Steg et al reported that revascularized patients in Killip class >I presented a 50% reduction in mortality at 6 months compared to nonvascularized patients with CHF.20 As expected, in the patients for whom LVEF values were available (69%) in this series, the percentage of subjects in Killip class >I and with an LVEF ≤40% was 48.7% compared to only 20% of patients without CHF.
Thus, and on the basis of the ischemic cascade,33 we suggest that the presence of SD in patients with NSTEACS identifies patients with greater and more severe coronary disease and, therefore, those who would obtain greater benefit from coronary revascularization.
Clinical Implications
Even though the current clinical practice guidelines recommend measuring the LVEF in the first hours of NSTEACS,1-2 data from large registries, such as the GRACE20 and CRUSADE5 registries, show that the measurement of LVEF is not routine. Similarly, the current risk assessment scales used more frequently do not include the presence of SD among their components. In view of our results, we believe that its rapid determination would add another step to risk stratification, identifying the patients who would obtain more benefit from an early RIS.
Limitations
Although these results are robust, there are a number of limitations that should be mentioned: a) the proportion of revascularized patients was less than that in large randomized studies; nevertheless, it is worth taking into account that our registry included consecutive unselected patients. Furthermore, the percentages of revascularization were similar to or even higher than those presented in other contemporary national and international observational studies5,20,34; b) we cannot rule out the possibility that our results could have been affected by residual confounding factors or, more importantly, by the lack of adjustment for known but unavailable covariates; c) the present results were obtained in a single center, which could limit, at least partly, the extrapolation of our results to other contexts; d) the limited number of patients with severe LVEF depression (LVEF≤35%) impedes our understanding of the true prognostic impact of coronary angiograph/revascularization in this subgroup; e) during the inclusion and follow-up periods the treatment guidelines underwent substantial changes, and thus we cannot rule out that this may have acted as a residual confounding factor that could have affected the present results; and f) given that systolic function was not routinely determined in early death, our data can only be extrapolated to patients who survived the hospital phase.
CONCLUSIONS
In patients with high-risk NSTEACS, the routine identification of patients with SD may be very useful for identifying those individuals who would benefit, in terms of long-term death or myocardial infarction, from routine coronary angiography. Future randomized studies are needed to confirm the present results.
ABBREVIATIONS
AMI: acute myocardial infarction
LVEF: left ventricular ejection fraction
NSTEACS: non-ST-segment elevation acute coronary syndrome
RIS: routine invasive strategy
SD: systolic dysfunction
SEE ARTICLE ON PAGES 888-9
This study was financed by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, Red HERACLES, RD 06/0009/1001 (Madrid, Spain)
Correspondence: Dra. P. Palau
Servicio de Cardiología. Hospital Clínico Universitario. Avda. Blasco Ibáñez, 17. 46010. Valencia. España.
E-mail: patricia.palau@hotmail.com
Received November 16, 2009.
Accepted for publication March 11, 2010.