Cardiac resynchronization therapy (CRT) is clearly indicated in heart failure patients in a higher functional class who have a severely depressed ejection fraction and wide QRS as a marker of interventricular or intraventricular asynchrony. It has been shown in this population that CRT improves symptoms, exercise capacity and ventricular function and reduces the number of hospitalizations as well as mortality.1,2 The classic criterion to establish asynchrony is QRS >120-150 ms. However, this parameter is not optimum for predicting CRT outcome, since approximately one-third of all patients who receive a resynchronization device do not show clinical echocardiographic improvement after device activation. This datum, confirmed in several series, indicates that the selection criteria used in most CRT studies (duration of QRS >120 ms, ejection fraction <35%, and New York Heart Association functional class III/IV heart failure) leads to the inclusion of a population which is too heterogeneous to optimally separate patients who respond to CRT from those who do not. For this reason, various measures have been introduced based on echocardiographic indexes and advanced Doppler ultrasound processing as an adjunct or alternative to using QRS duration to estimate the degree of asynchrony.3-7
However, the mechanical deformation process in these patients is very complex and presents great regional differences, making its evaluation difficult. In fact, routine echocardiographic techniques show great inter-observer variability and, up to now, low sensitivity and specificity and it has not been demonstrated that they significantly improve patient selection compared to QRS duration.8,9
In addition, the success of the technique depends on good patient selection as well as careful selection of lead location for left ventricular pacing.10 The ideal location should enable optimal resynchronization and have an appropriate pacing threshold. Furthermore, it should be accessible through the coronary venous system, whose anatomy is very variable, and should enable stable implantation in the absence of phrenic nerve capture. In this situation, the search for more sensitive and specific predictors of estimating CRT outcome and assessing the optimal lead location for left ventricular pacing have become a high priority issue within the fields of basic and clinical research. In this issue of the Revista Española de Cardiología, García Seara et al11 present an observational study on the association between frontal plane QRS axis and response to CRT in 78 patients implanted with a resynchronization device due to standard clinical indications. All were in functional class III or IV and 40% presented ischemic etiology. There were conduction abnormalities with left bundle branch block (LBBB) morphology in 71 (91%) of the patients, with a mean QRS duration of 170 ms. Most (71%) were in sinus rhythm. Placement of the left ventricular lead was attempted in the lateral or posterolateral veins of the coronary sinus, reserving the anterior interventricular vein in the event of failure. A combined criterion was employed to identify positive responders (improvement in functional class by at least 1 grade, an increase in left ventricular ejection fraction >5%, no need for hospitalization due to heart failure and the patient still being alive at 12-month follow-up). Both the implantation procedure and predicted treatment outcome were reasonably in line with standard practice, and thus the sample can be considered representative of the population receiving resynchronization therapy in Spain. In the univariate analysis, the authors did not find a significant association between left QRS axis deviation (less than -30o) before implantation and response to CRT. However, if only the patients with the lead implanted in the anterior interventricular vein are taken into account, the percentage of responders was greater when the patient had left QRS axis deviation (12 patients, 80%) than when it was normal (8 patients, 44%). In the multivariate analysis, a significant association was found between the QRS axis before implantation and lead location in predicting the outcome of CRT, and this was independent of other variables such as age, ejection fraction before implantation and the etiology of heart failure or mitral valve disease. The study has several limitations, basically due to the small sample size, its observational character, and the lack of techniques complementary to the electrocardiogram that offer more detailed information on the ventricular activation sequence. Despite this, the results are interesting because, although it appears reasonable to assume that the QRS axis contains information on the ventricular activation sequence, its potential relationship to the results of CRT have never been reported.
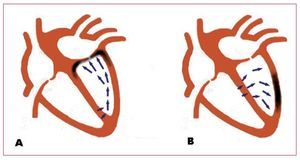
In patients with LBBB, left QRS axis deviation is not associated with the demographic variables or the etiology of the heart failure and, unlike prolonged QRS duration, neither does it seem to be associated with a greater degree of ventricular dysfunction.12 Some mechanisms that could explain QRS axis deviation include a greater degree of left anterior fascicular block, the loss of inferiorly directed forces due to fibrosis or scarring, preferential conduction in the left ventricular free wall secondary to anisotropy due to the myopathic process or extreme right ventricular hypertrophy and dilatation.13,14 The authors propose a hypothesis based on regional conduction delay to explain the interaction between the QRS axis before implantation and lead location on the effect of resynchronization. Left QRS axis deviation would represent activation from the lower area of the left ventricle to the upper, which would involve a longer delay in the anterior basal area, and thus better resynchronization would be obtained by stimulation in the anterior interventricular vein or in its collateral vessels. In contrast, in patients with a normal axis, the activation vector would be inferiorly directed, which means that the lead location in the posterolateral veins would obtain good ventricular synchrony (Figure). Although this hypothesis is very sound in relation to LBBB in a structurally normal or almost normal heart, but no data are available to allow extrapolation to the patients likely to receive a resynchronization device. The activation pattern in patients with heart failure and LBBB is complex and heterogeneous due to the functional lines of block that delay and divert the activation front and that are located in different areas depending on the patient.15,16 The presence of scarring can further complicate this pattern adding permanent areas of electrical inactivity to the functional lines of block. The sensitivity and specificity of QRS morphology are unknown in relation to the surface electrocardiogram in order to predict the ventricular activation sequence in this population. Although it may be thought that the information provided is limited, it addresses an issue that has barely been explored and whose interest is unquestionable. In this regard, the potential value of the QRS axis before implantation and its interaction with lead localization to predict the effect of CRT is an intriguing finding that deserves detailed study in a broader series. Currently, invasive techniques (electroanatomic mapping with catheter, endocardial noncontact activation mapping) and noninvasive techniques (electrocardiographic imaging and magnetocardiography) are available for the detailed study of endocardial and epicardial ventricular electrical activation in three dimensions. This information could be very useful in understanding the relationships between QRS morphology, the activation pattern, and the effect of resynchronization, at least in its electrophysiological aspect. The development of mathematical models that combine myocardial geometry, activation and contraction could also be useful to integrate the information obtained with various techniques. The medium-term objective would be to acquire detailed knowledge on activation in each patient which would help to predict the degree of efficacy of CRT and the best lead location to obtain the optimal hemodynamic effect, which would enable "made-to-measure" resynchronization, at least in its electrophysiological aspect. The superiority of this strategy compared to the current one must be demonstrated, but a priori it seems sufficiently promising to make the research effort required worthwhile.
Figure. Diagram of ventricular activation vectors in left bundle branch block with left QRS axis deviation (A) and normal QRS axis (B). A: depolarization advances from the inferoposterior area upwards, and thus the latest activation would correspond to the anterior basal region (darker area in the figure). B: the vectors advance toward the lateral and lower area, and thus lead location in the darker area would obtain good resynchronization.
SEE ARTICLE ON PAGES 1245-52
Dr García Alberola has participated in research projects financed by Boston and Medtronic.
Correspondence: Dr. A. García Alberola.
Servicio de Cardiología. Unidad de Arritmias. Hospital Universitario Virgen de la Arrixaca.
Ctra. de Cartagena s/n. 30120 El Palmar. Murcia. España.