Keywords
INTRODUCTION
In-stent restenosis has long been considered the main limitation hampering the long-term efficacy of coronary stenting. However, use of drug eluting stents, such as sirolimus eluting stents (Cypher) and paclitaxel eluting stents (TAXUS), are associated with markedly reduced restenosis rates in several randomized trials and registries.1-4 Drug eluting stents are now used in a majority of intracoronary stenting procedures. Nevertheless, in-stent restenosis still occurrs in some patients and some specific problems, such as late thrombosis after discontinuation of antiplatelet therapy has been reported.5
Recently, other approaches to treat the coronary artery disease have been introduced. Percutaneous approaches that use the adjacent venous circulation to bypass an obstructed artery and stent-based approach for ventricle to coronary artery bypass are being tested. Therapeutic angiogenesis and myogenesis are also being tested. Ongoing research on angiogenetic gene therapies and stem-cell transfer may result in additional therapeutic options benefiting patients with myocardial ischemia.
Despite the advances in the treatment of patients with coronary artery disease, sudden cardiac death is still prevalent. Sudden cardiac death occurs in half of all cardiac deaths and plaque rupture is the cause in more than 70% of such deaths.6,7 In addition, silent plaque rupture and its consequent wound healing accelerate plaque growth and is a more frequent feature in arteries with less severe luminal narrowing.8
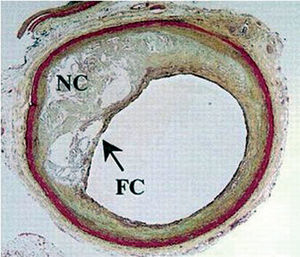
It has been established that atherosclerotic plaque composition is a key determinant of the fate of such lesions.9 Lipid-rich necrotic cores occupying a large percentage of the plaque are considered highly thrombogenic and prone to rupture.9,10 In addition, high levels of free-cholesterol and cholesterol clefts are common features of ruptured plaques and its most prevalent predecessor, the thin-cap fibro atheroma (TCFA) (Figure 1).6,11
Figure 1. Thin-cap fibro atheroma (TCFA). TC indicates thin fibrous cap; NC, Large necrotic lipid core.6 (Reprinted with permission of Lippincott Williams & Wilkins.)
A previous study of a large series of sudden death patients showed the presence of ruptured TCFA as the underlying cause of 60% of acute thrombi. Furthermore, 70% of those patients presented additional TCFAs without overlying rupture.12 Several novel invasive imaging techniques have been developed with the intention to identify one of more features of TCFA.13 Such techniques target the main characteristics of TCFA lesions: large lipid core, thin cap (≤65 μm) and positive remodeling. Detection of these non-obstructive, lipid rich, high-risk plaques may potentially have an important impact in the prevention of acute myocardial infarction and sudden death.
CATHETER-BASED CORONARY BYPASS
Percutaneous in Situ Coronary Venous Arterialization
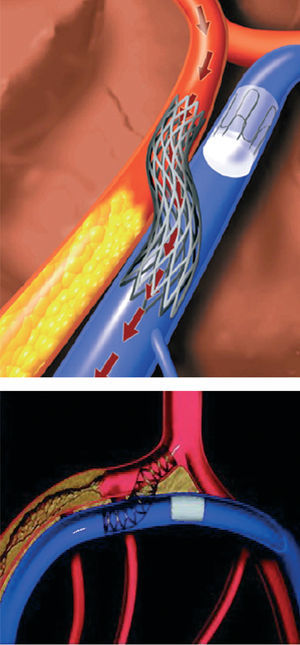
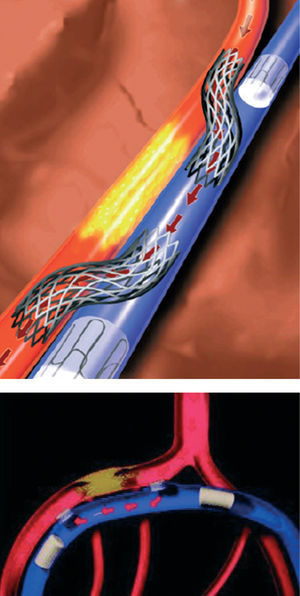
Catheter based coronary bypass has evolved since 1995.14,15 Percutaneous approaches that use the adjacent venous circulation to bypass an obstructed artery are being tested. In percutaneous in situ coronary venous arterialization (PICVA), a coronary artery is connected to the adjacent vein at one site upstream from the lesion, directing oxygenated blood flow into the vein (Figure 2). The oxygenated blood then travels through the venous system in the reverse direction to perfuse the myocardium. In a percutaneous in situ coronary artery bypass (PICAB), 2 channels are created between the coronary artery and the adjacent vein, one upstream and the other downstream from the lesion (Figure 3). The blood enters the upstream channel, flows through the isolated vein to bypass the lesion, and re-enters the healthy segments of the artery through the downstream channel. Oesterle et al reported the initial clinical experience with PICVA.15 PICVA was attempted in eleven patients with severe angina and no reasonable option for either angioplasty or surgical revascularization. In 6 patients, the adjacent vein could not be adequately targeted for successful needle and wire delivery. PICVA was successfully completed in 5 patients. Two of the 5 cases had catastrophic complications and died within 48 hr of the procedure. Of the remaining 3 cases, all patients experienced an improvement in their anginal symptoms. However, 3-month follow-up angiography revealed a closed PICVA channel in 2 patients. After this initial clinical experience, the system of devices is currently undergoing significant modification (the new imaging strategy and modified connecting and blocking devices), and a further clinical study is underway.
Figure 2. Percutaneous in situ coronary venous arterialization (PICVA).15 (Reprinted with permission of John Wiley & Sons Ltd. All Rights Reserved.)
Figure 3. Percutaneous in situ coronary artery bypass (PICAB).15 (Reprinted with permission of John Wiley & Sons Ltd. All Rights Reserved.)
Stent-Based Approach for Ventricle to Coronary Artery Bypass
Alternative approach, also performed via sternotomy, is a ventricle to coronary artery bypass (VCAB). In this procedure, a stent-based device (VSTENT) is used to create a conduit between the left ventricle and the left coronary artery, thereby increasing flow in the coronary artery. The advantage of this approach is that no grafting is necessary and it can be performed rapidly. Only one experimental revascularization procedure has been reported.16
RETROGRADE PERFUSION
Synchronized retroinfusion with arterial blood to coronary veins was able to partially reduce myocardial ischemia in patients undergoing percutaneous coronary angioplasty (PTCA).17 In general, patients with left main stenosis or left main-equivalent stenosis are considered to be at high risk for PTCA. Pohl et al reported on the 1-year results of a prospective randomized single center study (Myoprotect I) in 44 patients with symptomatic left main or left main-equivalent lesions, who were randomly assigned to the stent group (n=23) or the bypass group (n=21).18 In all patients randomized to percutaneous treatment, selective pressure-regulated retroperfusion of arterial blood into the anterior cardiac vein was applied during ischemia. Twenty-eight-day mortality and 1-year mortality rate as well as quality of life scores were similar in both groups. Though event free survival was lower and target lesion revascularization rate was higher in the stent group, retroperfusion-supported stent implantation was associated with substantially lower costs and might be considered as an alternative treatment option in the selected group of high-risk patients.
THERAPEUTIC ANGIOGENESIS AND VASCULOGENESIS
Chronic imbalances of myocardial oxygen supply and demand produced by a coronary artery stenosis or occlusion have been shown to increase growth of the coronary collateral circulation. Angiogenesis and vasculogenesis are adaptive responses of the coronary collateral circulation to myocardial ischemia.
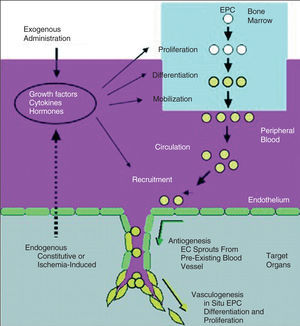
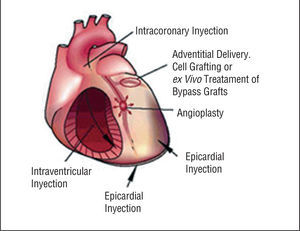
Therapeutic angiogenesis and vasculogenesis, which involves the administration of angiogenic growth factors, cytokines or stem cells to stimulate collateral formation and improve myocardial perfusion, is being tested as an alternative strategy for patients with medically intractable angina who are not candidates for mechanical revascularization therapies (Figure 4).
Figure 4. Growth factors, cytokines, or hormones released endogeneously in response to tissue ischemia, or administered exogenously for therapeutic neovascularization, act to promote endothelia progenitor cell (EPC) proliferation, differentiation, and mobilization from bone marrow (via the peripheral circulation) to neovascular foci.19 (Reprinted with permission of Journal of Clinical Investigation.)
Angiogenic Cytokines
Preclinical studies have established a foundation for rational development of therapeutic angiogenesis.20-23 A variety of growth factors and chemokines convincingly increase the formation of small blood vessels in experimental models. Most clinical trials to date involve transfer of vascular endothelial growth factor (VEGF) or fibroblast growth factor (FGF) by several delivery strategies (Figure 5).24
Figure 5. Clinical feasible gene delivery routes.25 (Reprinted with permission of Nature Publishing Group.)
Protein Transfer
With the advent of recombinant DNA technology, large quantities of purified proteins can be produced for therapeutic use (Table 1).25 Advantages of locally administering purified angiogenic factors include easy dose titration, repeat administration if necessary, and rapid metabolism to prevent toxicity.25 The primary disadvantages have been either a lack of efficacy in placebo-controlled studies or a need for administration during coronary artery bypass graft (CABG). Methods combining angiogenic proteins with slow-release systems are in development.
Gene Therapy
Gene therapy is an approach in which the genetic material directing production of a protein is transferred in place of the protein itself. A number of vehicles are used for transferring DNA to heart tissue, including purified DNA, DNA/lipid complexes, adeno-associated viruses, or adenovirus.24,25 An advantage of this approach appears to be localized, sustained but not indefinite production of angiogenic factors.26 Apparent disadvantages are the possibilities of vector toxicity, an immune response to the gene therapy vector, or inappropriately localized gene transfer resulting in angiogenesis in a tumour or the retina.
The efficacy of gene transfer approaches to therapeutic angiogenesis is now being tested in clinical trials (Table 2).25,27-33 Early uncontrolled open-label clinical trials generally gave positive results, although the possibility of a placebo effect was not excluded. Controlled Phase II trials are providing positive but not definitive results. This is promising, since the patient population being studied has failed all other therapies and is likely to be refractory to intervention. However, most of the efficacy measures studied to date are surrogate endpoints such as exercise tolerance time (ETT), angina, or perfusion. While these measures are useful in suggesting clinical efficacy, hard clinical endpoints such as mortality, myocardial infarction (MI) and the need for revascularization should be studied. Long-term follow up data are also necessary. An important observation is that the safety results of these trials indicate no major problems. Potential side-effects such as worsening of atherosclerosis, retinopathy or cancer, have not been observed in clinical trials.
Two large Phase III clinical trials (AGENT 3 and 4) were designed to evaluate further the safety and efficacy of Ad5FGF4.31 Both trials were designed as randomized, double blind and placebo controlled trials. In each trial, a recruitment goal was 450 patients and these patients would be randomized to 3 groups (placebo group, Ad5FGF-4 at a dose of 109 vp group, and Ad5FGF-4 at a dose of 1010 vp group). In January 2004, enrollment was stopped (416 patients in AGENT 3 and 115 patients in AGENT 4) because interim data analysis of AGENT 3 indicates that the studies will provide insufficient evidence of efficacy. However, enrolled patients' follow-up continues and final data will be presented in the near future.
Cell Based Therapy
Another alternative method for increasing coronary vascularization is the transplantation of stem or progenitor cells.34,35 These cells not only produce a variety of growth factors and cytokines, but participate structurally in the formation of new vascular tissue and myocyte.
The promising results from experimental studies promoted the initiation of clinical trials.36,38 Stem and progenitor cells are being tested in patients with both acute myocardial infarction and chronic ischemic heart failure (Table 3 and 4).35,39-46 Improved wall motions or increased perfusion was demonstrated in most of study patients. However, BOOST is the sole randomized controlled clinical trial.42 Sixty patients with ST-segment elevation myocardial infarction were randomly assigned to either a control group that received optimum post infarction medical treatment, or a bone-marrow-cell group that received optimum medical treatment and intracoronary transfer of autologous bone marrow cells 4.8 days after percutaneous coronary intervention (PCI). After 6 months, mean global left ventricular ejection fraction (LVEF) had significantly increased in the bone-marrow-cell group, compared to the control group. However, most studies at present are limited by the small patient enrolment, and recently some papers reported that bone-marrow-derived haematopoietic stem cells do not transdifferentiate into cardiac myocytes in ischemic myocardium.47,48
PERCUTANEOUS MYOBLAST TRANSPLANTATION
Most preclinical experience has been reported on transplantation of skeletal myoblasts in infarcted myocardium.49-52 These studies demonstrated that transplanted skeletal myoblasts in damaged myocardium are capable of cellular engraftment, myotube formation, and long-term graft survival. Several clinical percutaneous myoblast transplantation trials, using both the transendocardial and the transvenous approaches, have been reported.
Transendocardial Approach
A pilot safety and feasibility study on percutaneous transplantation of autologous skeletal myoblasts by transendocardial injection in five patients with ischemic heart failure was reported by the group of Rotterdam.50 Autologous skeletal myoblasts were obtained from the quadriceps muscle and cultured in vitro for cell expansion. With a NOGA®-guided catheter system, 196±105 million cells were transendocardially injected into the infarcted area. After 3 months, LV ejection fraction increased and regional wall analysis by magnetic resonance image (MRI) showed significant increased wall thickening at the target area. In the light of these preliminary favourable results, a multicenter European uncontrolled investigation has been started. Overall, 15 patients have been enrolled and treated with transendocardial skeletal myoblasts injection. The results of this study will be presented in the near future.
Transvenous Approach
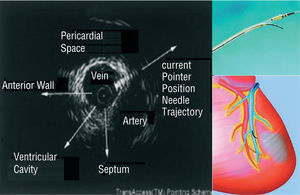
A novel catheter-based endovascular system for direct myocardial injection using IVUS guide needle punctures via the coronary venous system (the TRANSAccessTM) has been developed (Figure 6).53 In the POZAN trial, 10 patients underwent intramyocardial injection by using the TRANSAccess.54 The procedure was not successful in one patient but NYHA class improved in all patients and ejection fraction increased 3%-8% in 6 out of 9 cases during 6 month follow-up.
Figure 6. Direct myocardial injection using IVUS guide needle punctures via the coronary venous system.
Ventricular Arrythmia
After intramyocardial injection of skeletal myoblasts, ventricular arrhythmia was reported. Menasche et al52 reported that implantable cardioverter-defibrillator (ICD) implantation for ventricular arrythmias was required in 4 out of 10 patients after open chest autologus myoblast transplantation.50,52 One of the 5 patients who were enrolled in the study on percutaneous transplantation of autologous skeletal myoblasts by transendocardial approach had also sustained episodes of ventricular tachycardia and required ICD. This is probably related to: a) heterogenecity of action potentials between the native and the transplanted stem cells; b) intrinsic arrhythmia potential of injected cells; c) increased nerve sprouting induced by stem cell injection; and d) local injury or edema induced by myocardial puncture and inflammatory response.
FUTURE PERSPECTIVES TOWARDS INVASIVE DETECTION OF VULNERABLE PLAQUE
Lipid-Rich Atheromatous Core
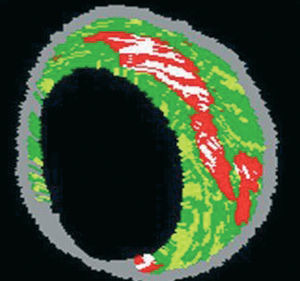
IVUS provides an accurate tomographic view of the coronary arteries and has shown a high correlation with histology samples in in vitro validation studies.55-57 However, accurate plaque characterization with visual interpretation of gray-scale IVUS, particularly of lipid rich plaques, remains an unresolved issue.56 On the contrary, spectral analysis of IVUS radiofrequency data (IVUS-Virtual HistologyTM [IVUS-VH]) has demonstrated potential to provide detailed quantitative information on plaque composition and has been validated in studies of explanted human coronary segments (Figure 7).58
Figure 7. Virtual histology image. Red: lipid core. White: calcium. Yellow: fibrofatty. Green: fibrosis. Gray: media.
Angioscopic yellow plaques have been related to atheromatous plaques in previous validation studies.59 However, this technique evaluates only the luminal surface of the intima thus quantifiable values of lipid core size are out of its scope.
Raman spectroscopy is a technique that can characterize the chemical composition of tissues.60 In vitro studies have demonstrated that diagnostic algorithms allow the discrimination of coronary arterial tissue in 3 categories: non-atherosclerotic, non-calcified and calcified plaques.61 Nevertheless, this technique also lacks the ability to provide geometrical information of the vessel and has a shallow penetration depth.
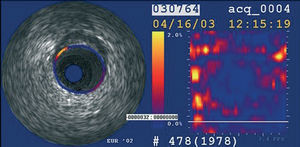
Intravascular ultrasound elastography and palpography are techniques that allow the assessment of local mechanical tissue properties (Figure 8).62,63 At a defined pressure, soft tissue (lipid-rich) components will deform more than hard components (fibrous-calcified).64 However, they are unable to provide quantifiable measurements of plaque components.
Figure 8. Palpography image. Yellow, high strain spot.
Finally, intravascular magnetic resonance (IVMRI) has recently emerged as a potential tool to identify TCFA since it can accurately determine the presence of lipid within the arterial wall.65,66 In vivo feasibility remains to be proven and acquisition time and extent of the scan analysis requires improvement.
Thin-Fibrous Cap
The threshold to define the cap as "thin" has previously been set at <65 μm.67 However, it is well established that general tissue shrinkage can not be avoided during dehydration.68 Shrinkage of up to 60%, 15% and 80% can occur during critical-point-drying, free-drying, and air-drying respectively.69 Furthermore, post-mortem contraction of arteries further contributes to deteriorate the pathological quantification of atherosclerosis.70 Accordingly, we believe that the threshold should be higher than 65 μm. Since the axial resolution of IVUS is between 100 and 150 μm, techniques such as IVUS-VH with the capacity to quantify each plaque component and to identify the location of the lipid core in relation to the lumen have the potential to recognize all the 3 features of TCFA lesions.
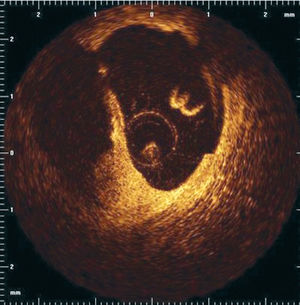
Optical coherence tomography (OCT) is an imaging technique that allows high-resolution (axial resolution of 15 μm) imaging in biological systems (Figure 9).71 Accordingly, OCT has the capacity to allow in-vivo, real time visualization of a thin fibrous cap. Recent in vivo data suggest the possibility of detection of macrophages in atherosclerotic plaques.72
Figure 9. OCT image after plaque rupture.
The sensitivity and specificity of palpography to detect vulnerable plaques has recently been assessed in post-mortem human coronary arteries where vulnerable plaques were detected with a sensitivity of 88% and a specificity of 89%.63
Positive Remodeling
The expansive compensatory enlargement of the coronary arteries in response to an increase in plaque area is called positive or expansive remodeling.73 Several studies showed an increase in inflammatory marker levels, larger lipid cores, and pronounced medial thinning in positive remodeled vessels.74-76 Recently, the relationship between vascular remodeling and plaque composition was assessed using IVUS.77-79 In these studies, the remodeling index for soft lesions was significantly higher than those for fibrous/mixed and calcified lesions.77-79 Aside from gray-scale IVUS, IVUS-VH can also accurately identify, as well as lipid core size and TCFA, the geometry of the vessel.
Pan-Coronary Syndromes
Several studies using angiography, IVUS, angioscopy, and palpography identified a high incidence of high-risk plaques throughout the coronary tree.80-83 In the study conducted by Rioufol et al,81 at least one plaque rupture was found away form the culprit lesion in 80% of the patients, away from the culprit artery in 71% and in the 2 non-culprit arteries in 12.5%.81
Patients with acute myocardial infarction may present additional complex non-infarct-related coronary plaques. Furthermore, such multiple complex plaques have been found independent predictors of future clinical events.80
If the large number of high-risk plaques is detected throughout the coronary tree by means of angiography, angioscopy, IVUS, and palpography potential local preventive strategies could not be cost-effective.8,80-83 On the contrary, a systemic "plaque stabilization" approach including statins or angiotensin converting enzyme (ACE) inhibitors could be capable of "cooling-down" the inflammatory burden.84
CONCLUSIONS
Numerous percutaneous interventional treatments and diagnostic tools have been developed to diagnose the vulnerable plaque and to treat the large and increasing number of patients with myocardial ischemia. The proliferation of these strategies, and the need for multiple approaches in individual patients indicate that the problem of myocardial ischemia is not solved. Ongoing research on the use of drug eluting stents, catheter based bypass graft, therapeutic angiogenesis and myogenesis, and the catheter based devices to detect the plaque vulnerability and composition may result in additional diagnostic and therapeutic options for patients with coronary artery disease.
Section Sponsored by Laboratorio Dr Esteve
Correspondence: P.W. Serruys MD, PhD.
Thoraxcenter, Bd 406. Erasmus MC, Dr Molewaterplein 40. 3015 GD Rotterdam. Países Bajos.
E-mail: p.w.j.c.serruys@erasmusmc.nl