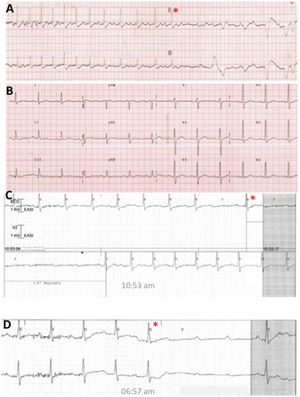
We present the case of a male patient, a 45-year-old cattle farmer, with presyncopal and syncopal episodes; the last of these events resulted in head trauma. In addition, he had paroxysmal atrioventricular block (AVB) with prolonged pauses, always at rest (figure 1, asterisk). Given his age and the high number of sudden-onset syncopal episodes, we considered that echocardiography and magnetic resonance imaging would be valuable (despite not being specifically recommended in clinical practice guidelines). These techniques ruled out structural heart disease and signs of sarcoidosis. Due to his occupation, we also tested for Lyme disease, which was negative. Adequate tachycardization was seen with exercise (during telemetry monitoring), as well as a syncopal (cardioinhibitory) response in the tilt table test. The proposed management was autonomic modulation via radiofrequency ablation of the ganglionated plexi.
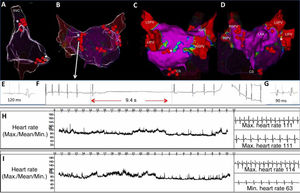
The procedure was performed under mild sedation. Electroanatomical mapping (Biosense Webster, United States) of the left and right atrium (RA) performed with a multipolar catheter (figure 2A-D) failed to identify any notable low-voltage (< 0.5mV) areas. The patient had a baseline AH interval of 120 milliseconds (figure 2E). The multipolar catheter was then replaced with an ablation catheter, used to apply high-frequency pacing (10-second bursts at 20Hz for 25mA/ms) in the region of the ganglionated plexi to identify vagal responses. Because this procedure was sometimes complicated by the induction of atrial fibrillation, anatomical sites of possible relevance were ablated (in the RA, the junction of the superior vena cava [SVC] with the RA in the posterior region and the coronary sinus [CS] ostium [figure 2A]; in the left atrium, the anterosuperior antral region of the right superior pulmonary vein [contralateral to the SVC application] and the left superior pulmonary vein [figure 2B], the posteroinferior region of the left inferior vein [figure 2C], and the area of the ligament of Marshall at its insertion into the CS [figure 2D]). During the radiofrequency ablation, prolonged asystoles were sometimes obtained, up to 9.4seconds (figure 2F). The AH interval after the ablation was 90 milliseconds (figure 2G) and there was no notable change in the PP interval (or in the Wenckebach point). After the procedure, the patient remained hospitalized with electrocardiographic monitoring (for 72hours) and no AVB episodes were recorded. At 1 month, there were still no blocks in Holter monitoring (but a slight variability in heart rate [HR], with a minimum rate of 66 bpm) (figure 2H) and an atropine test showed appropriate denervation, given the absence of tachycardization. Holter ECG results at 4 months were almost identical to the previous test results (minimum HR of 62 bpm, 0 pauses <2.5 s) (figure 2I). The patient also showed considerable clinical improvement, with no presyncope or syncope and an adequate response to physical exercise.
A: anatomical reconstruction of the right atrium (RA) showing the radiofrequency applications at the junction of the superior vena cava (SVC) with the RA (asterisk) and at the coronary sinus (CS) ostium (star). B: anterior view of the left atrium mainly showing the lesions contralateral to the SVC in the anterosuperior region of the right pulmonary vein (asterisk). C: posterior view of the left atrium showing the 4 pulmonary veins (PVs): left superior (LSPV), left inferior (LIPV), right superior (RSPV), and right inferior (RIPV). D: anterior view (slightly oriented toward the left) showing the applications in the region of the ridge between the left atrial appendage (LAA) and the left PVs (asterisk). E: AH interval before ablation. F: asystole provoked by radiofrequency application in the anterosuperior region of the RSPV. G: AH interval after ablation. H and I: Holter-ECG frequency histograms obtained 1 and 4 months after ablation.
In this case, endocardial ablation of the ganglionated plexi is presented as an alternative to pacemaker implantation. Various groups have reported the results of biatrial ablation1 or even unifocal ablation in the RA2 in patients with neurally mediated syncope and severe cardioinhibitory response. Although numerous doubts remain regarding the long-term outcomes (eg, only 48 patients in the series reported by Qin et al.1 and 9 in that reported by Debruyne et al.2 completed 12 months of follow-up) and the optimal strategy, this approach currently represents a promising alternative for highly selected patients such as ours. Although permanent pacemaker implantation would have helped to control the AVB episodes in our patient, given the positive response to the tilt table test, we believe that there was a high probability of syncopal recurrence due to the persistence of the vasodepressive response.3,4 Theoretically, the interruption not only of the efferent pathway with endocardial ablation, but also that of the afferent pathway might underlie the amelioration of both the hypotensive and cardioinhibitory responses. In addition, the slight increase in HR after the cardiomodulation might increase cardiac output, which, as previously described,4 represents a more relevant mechanism to the syncopal response than the decrease in peripheral vascular resistance.