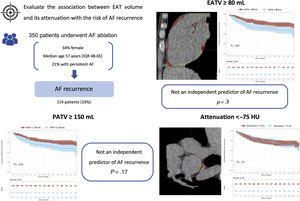
Previous studies have suggested that epicardial adipose tissue (EAT) could exert a paracrine effect in the myocardium. However, few studies have assessed its role in the risk of atrial fibrillation (AF) recurrence. This study aimed to evaluate the association between EAT volume, and its attenuation, with the risk of AF recurrence after AF ablation.
MethodsA total of 350 consecutive patients who underwent AF ablation were included. The median age was 57 [IQR 48-65] years and 21% had persistent AF. Epicardial fat was quantified by multidetector computed tomography using Syngo.via Frontier-Cardiac Risk Assessment software, measuring pericardial fat volume (PATV), EAT volume, and attenuation of EAT posterior to the left atrium. AF recurrence was defined as any documented episode of AF, atrial flutter, or atrial tachycardia more than 3 months after the procedure.
ResultsAfter a median follow-up of 34 [range, 12-57] months, 114 patients (33%) had AF recurrence. Univariable Cox regression showed that patients with an EAT volume ≥ 80mL had an increased risk of AF recurrence (HR, 1.65; 95%CI, 1.14-2.39; P=.007). However, after multivariable adjustment, EAT volume did not remain an independent predictor of AF recurrence (HR, 1.24; 95%CI, 0.83-1.87; P=.3). Similar results were observed with PATV. Patients with lower attenuation of EAT did not have a higher risk of AF recurrence (log-rank test, P=.75).
ConclusionsEAT parameters including the evaluation of EAT volume, PATV and EAT attenuation were not independent predictors of AF recurrence after catheter ablation.
Keywords
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in adults and carries substantial morbidity and mortality.1 In addition, it represents a major clinical challenge due to its predisposition to relapse even after AF ablation.1
It is known that obesity is one of the most important risk factors for the development of AF.2 Several pathophysiological mechanisms can be responsible for this association including a systemic, endocrine, or paracrine (local) effect.2,3 Epicardial adipose tissue (EAT), defined as fat between the myocardium and pericardium, is a metabolically active tissue that produces several proinflammatory cytokines that can have a direct effect on the myocardium.4
Previous studies have shown that EAT is independently associated with a higher coronary atherosclerotic burden5,6 and can adversely influence cardiac remodeling, causing left atrial (LA) dilation, left ventricular hypertrophy and diastolic dysfunction, and increasing risk of heart failure with preserved ejection fraction.7–9
However, few large studies have evaluated the influence of EAT with the risk of AF or its recurrence.10,11 Moreover, there are several methods to quantify the amount of EAT, which might affect results. For example, previous studies have suggested that the amount of EAT posterior to the LA could be associated with AF burden and recurrence, but, in these studies, EAT thickness was measured as the shortest distance between the mid-LA and esophagus.12,13 The quantification of total EAT volume could be a more reliable measurement of EAT, because arrhythmogenesis can be mediated by shared pathways.4 Volumetric assessment is the most accurate EAT volume measurement since thickness varies across cardiac regions.4
Mechanistic studies have suggested that an inflammatory process related to EAT can contribute to arrhythmogenesis.4 Earlier studies have shown that fat attenuation measured by computed tomography (CT) correlates with inflammation detected by histologic examination and positron emission tomography scan,14 being a potential marker of EAT local inflammation. This measurement has been linked to acute coronary syndrome14,15 and more recently some authors have explored its role in AF ablation outcomes.16,17
In this study, we aimed to evaluate the association between EAT and the risk of AF. Specifically, we aimed to assess if EAT volume could be a predictor of AF recurrence after AF catheter ablation, and if the attenuation of EAT posterior to the LA (a possible biomarker of local inflammation) could be involved in this association.
METHODSStudy populationBetween January 2017 and December 2019, all consecutive patients with symptomatic AF who underwent CT for procedure planning were included in this retrospective cohort study. Patients were excluded if they had coronary artery disease or moderate-severe valvular heart disease. Baseline demographic, clinical and outcome data were gathered from medical records. The definition of the different variables is listed in .
The study was conducted following the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee (internal reference 191-21). Informed consent for the use of clinical data were obtained before the performance of the CT scan.
Cardiac adipose tissue measurementsA multidetector CT scan was performed in all patients on the day of the procedure using a 384-slice (2x192) multidetector CT (SOMATOM Force Siemens) according to a standardized protocol (summarized in the supplementary data). All data were processed using a dedicated workstation (Syngo.via Frontier–Cardiac Risk Assessment, Siemens Medical Solutions).
Cardiac adipose tissue measurements were determined by investigators who were blinded to patient outcomes. Noncontrast acquisition was used for all measurements. Adipose tissue was defined in the range of−150 and−50 Hounsfield units (HU).
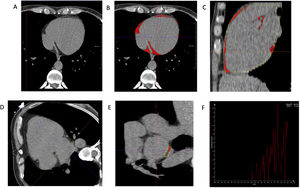
Cardiac adipose tissue was measured with 3 methods, as detailed in figure 1:
- •
Epicardial adipose tissue volume (EATV) was defined as fat between the outer wall of the myocardium and pericardium. For its assessment, the pericardium was manually contoured every 10mm from the pulmonary artery bifurcation to the level of the mitral valve and for every 20mm from there to the diaphragm, and then, was semiautomatically reconstructed as the sum of all slices, as illustrated in figure 1A-C. Manual adjustment was performed when necessary.
- •
Pericardial adipose tissue volume (PATV) was defined as fat internal and external to pericardium.4 Its value was automatically estimated by the validated software Syngo.via Frontier–Cardiac Risk Assessment (Siemens Medical Solutions).
- •
Attenuation of EAT posterior to the LA was measured in a single slice, in short-view axis, between the mid-LA and esophagus. For this selected area, values of attenuation for each point were displayed in a graph and attenuation was defined as the mean value, as illustrated in figure 1D-F.
Example of measurement of epicardial adipose tissue volume by CT (A-C). A: pericardium contour from the axial view. The pericardium was manually contoured from the pulmonary artery bifurcation to the diaphragm. Final volume was semiautomatically calculated as the sum of all slices. B and C: the red color indicates total EATV from the axial and sagittal vieww, respectively. Example of measurement of attenuation of fat posterior to the left atrium by CT (D-F). D: in 4-chamber view, the mid-LA was identified. E: in short-view axis, on a single slice, an area between the LA and esophagus was contoured. F: for the selected area, values of attenuation for each point were displayed on a graph. Attenuation was defined as the mean value.
Interobserver variability was assessed in 20 randomly selected patients. The interclass correlation coefficient obtained was 0.991 (P=<.001) for EATV measurement and 0.884 (P=<.001) for attenuation of EAT posterior to LA measurement.
Catheter ablation procedureAll procedures were performed under intravenous sedation with blood pressure, oxygen saturation, and electrocardiogram monitoring, with anesthesiologic assistance. Femoral venous access was used for all procedures. A decapolar catheter was placed in the coronary sinus. Transseptal puncture was performed under fluoroscopy guidance.
Catheter ablation procedures included pulmonary vein isolation with point-by-point radiofrequency ablation and second-generation cryoablation. Point-by-point radiofrequency ablation was performed using an irrigated 3.5-mm ablation catheter (Thermocool SmartTouch or Thermocool SmartTouch SF) guided by a 3D electroanatomic mapping system (CARTO-3, Biosense Webster Inc, USA).
An extensive encircling pulmonary vein isolation was performed. Concomitantly, cavotricuspid isthmus ablation was performed in selected patients with documented typical atrial flutter. Additional ablation, such as posterior wall isolation, linear lesions, nonpulmonary vein triggers, and low-voltage zone ablation, was also performed at the operator's discretion.
When cryoablation was performed, the Arctic Front Cardiac CryoAblation Catheter System, the second-generation Arctic Front Advance and the FlexCath Steerable Sheath (Medtronic, USA) were used; a 28-mm balloon was used for all patients.
The mapping catheter was positioned at the pulmonary veins ostium, where baseline potentials were documented. The balloon was inflated in the LA. Contrast was injected to assess balloon occlusion and, when achieved, cryothermal energy was applied. Freezing cycles were applied to each pulmonary vein for a minimum of 240seconds. Using decapolar catheter, diaphragmatic stimulation was performed to avoid phrenic nerve palsy.
The type of energy employed was chosen at the operator's discretion. The procedural endpoint was electrical isolation of all pulmonary veins from the LA. Electrical cardioversion was performed when AF persisted at the end of the ablation procedure.
Follow-upThe standard follow-up protocol used in our institution includes a first clinical evaluation and a 12-lead electrocardiogram performed 1 month after discharge. Then the patient is evaluated at 3 and 6 months with a 12-lead electrocardiogram and a telephone-based consultation with a cardiologist. Between 9 and 12 months after the ablation procedure, 24-hour ambulatory electrocardiogram monitoring is performed, followed by electrocardiogram and clinical evaluation at 12, 18, and 24 months, and then every 12 months. At each visit, patients are evaluated for the recurrence of arrhythmias with a standardized questionnaire for arrhythmia-related symptoms (palpitations, chest discomfort, fatigue, and dizziness), physical examination, and a 12-lead electrocardiogram. Additional patient visits may be needed according to clinical status.
Study endpointsThe primary outcome was defined as AF recurrence after the blanking period (more than 3 months after the procedure).18 Recurrence was defined as any documented (electrocardiogram/Holter) episode of AF, atrial flutter, or atrial tachycardia lasting more than 30seconds.18 Information about AF recurrence was collected by reviewing medical records and was censored in November 2021. We also collected information on total recurrence rate (secondary endpoint), defined as any documented episode after the ablation, including the blanking period.
Statistical analysisCategorical variables are expressed as absolute values and percentages. Continuous variables are expressed as mean±standard deviation (SD) for normally distributed data or median and interquartile range [IQR], for nonnormally distributed data. Patients’ characteristics were compared between patients with and without recurrence using the chi-square test or the Fisher exact test t-test or the Mann-Whitney U test, as appropriate.
Initial data exploration encompassed logistic regression with restricted cubic polynomial transformation to account for nonlinear relationships between AF recurrence and adipose tissue measurements (). Inspection of the logistic regression partial effect curves suggested that patients at risk of recurrence could potentially be stratified by a cutoff of 80mL for EAT volume, 150mL for PAT volume and−75 HU for EAT posterior to LA attenuation (). Cox proportional hazard models were used to estimate the effect of EAT measurements following 2 strategies. As a dichotomous variable (defined by the data-driven cutoffs defined above) and as a continuous value indexed to total body surface area. Both models were adjusted to clinically relevant covariates, age, sex, hypertension, diabetes, obesity, AF subtype, and dilated LA. Proportional hazards assumptions were assessed using Schoenfeld residuals. Hazard ratios (HR) with 95% confidence intervals (95%CI) and P values are reported and considered statistically significant if P <.05.
Cumulative recurrence-free survival for dichotomous EAT was estimated using the Kaplan-Meier method and were compared using the log-rank test.
All statistical tests and plots were done using R statistical software, version 4.1.2,19 together with the “rms”,20 “survival”,21 “survminer”,22 and “gtsummary”,23 packages.
RESULTSPopulation characterizationDuring the study period, 531 patients underwent AF ablation. Of these, 125 patients were excluded because they had coronary artery disease or moderate-severe valvular heart disease and 56 were lost to follow-up. The clinical, echocardiographic and ablation procedure characteristics of the study population are listed in table 1. Among the 350 patients included, 34% were female with a median age of 57 [IQR 48-65] years. Persistent AF was present in 21% and 12% had undergone a previous AF catheter ablation procedure.
Patient characteristics
| Overall populationN=350 | No AF recurrence,n=236 | AF recurrence,n=114 | P | |
|---|---|---|---|---|
| Sex, female | 119 (34) | 71 (30) | 48 (42) | .026 |
| Age, y | 57 [48-65] | 55 [47-63.75] | 61 [51.75-67] | .001 |
| BMI, kg/m2 | 27.4±3.83 | 27.3±3.82 | 27.5±3.87 | .599 |
| BSA, m2 | 1.93 [1.80-2.05] | 1.92 [1.81-2.05] | 1.94 [1.79-2.07] | .814 |
| Comorbidities | ||||
| Hypertension | 166 (47) | 110 (47) | 56 (49) | .659 |
| Diabetes mellitus | 41 (12) | 23 (10) | 18 (16) | .099 |
| Dyslipidemia | 153 (44) | 99 (42) | 54 (47) | .338 |
| Smoking: active | 40 (12) | 27 (12) | 13 (12) | .994 |
| Heart failure | 15 (6) | 10 (6) | 5 (7) | >.9 |
| Cerebrovascular disease | 6 (2) | 3 (1) | 3 (3) | .394 |
| Thyroid dysfunction | 66 (19) | 36 (15) | 30 (26) | .013 |
| Chronic renal disease | 34 (10) | 22 (9) | 12 (11) | .721 |
| OSA | 35 (10) | 26 (11) | 9 (8) | .362 |
| Pulmonary disease | 13 (4) | 9 (4) | 4 (4) | >.9 |
| Persistent AF | 75 (21) | 36 (15) | 39 (34) | <.001 |
| AF duration, y | 3 [1-6] | 2 [1-5] | 3 [2-6.5] | .031 |
| CHA2DS2-VASc | .043 | |||
| 0 | 98 (28) | 74 (32) | 24 (22) | |
| 1 | 118 (34) | 80 (34) | 38 (34) | |
| 2 | 79 (23) | 55 (24) | 24 (22) | |
| >2 | 50 (15) | 25 (11) | 25 (23) | |
| Previous cardioversion | 119 (36) | 72 (32) | 47 (44) | .042 |
| Previous pulmonary vein isolation | 41 (12) | 24 (10) | 17 (15) | .196 |
| Anticoagulation | 214 (65) | 139 (61) | 75 (73) | .041 |
| Antiarrhythmic drugs | 169 (59) | 113 (57) | 56 (62) | .474 |
| Beta-blockers | 214 (61) | 137 (58) | 77 (68) | .088 |
| Echocardiography parameters | ||||
| LA volume, mL/m2 | 35 [30-40] | 33 [30-39] | 37 [30-42] | .001 |
| LA diameter, mm | 40 [36-43] | 39 [36-42] | 41 [37-45] | .001 |
| Dilated LA | 194 (60) | 121 (55) | 73 (72) | .005 |
| LVEF | 60±4.6 | 60±4.6 | 60±4.5 | .829 |
| Procedural characteristics | ||||
| Ablation energy: radiofrequency | 254 (73) | 169 (72) | 85 (75) | .562 |
| Follow-up | ||||
| Early recurrence | 43 (13) | 9 (4) | 34 (30) | <.001 |
| Mortality | 1 (0.3) | 0 | 1 (0.9) | .326 |
AF, atrial fibrillation; BMI, body mass index; BSA, body surface area; LA, left atrium; LVEF, left ventricle ejection fraction; OSA, obstructive sleep apnoea.
The data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
During follow-up, 43 patients (13%) had AF recurrence within the first 3-month period. After the 3-month blanking period, 114 patients (33%) developed AF recurrence with a median follow-up of 34 months within the range of 12 to 57 months. Compared with those without recurrence, patients with AF recurrence were older, more frequently female, had significantly more thyroid dysfunction, persistent AF, longer disease duration, and a greater LA volume (table 1).
Relationship between EAT volume and AF recurrencePatients with an EATV ≥ 80mL had a higher risk of AF recurrence than patients with lower EATV (log-rank test P=.007), as shown in the Kaplan-Meir curves (figure 2A). Moreover, in the univariable Cox proportional hazards regression, patients with EATV ≥ 80mL were at higher risk of recurrence (HR, 1.65; 95%CI, 1.14-2.39; P=.007), as detailed in table 2. However, after multivariable adjustment (age, sex, hypertension, diabetes, obesity, AF subtype, and dilated LA), EATV ≥ 80mL did not remain an independent predictor of AF recurrence (HR, 1.24; 95%CI, 0.83-1.87; P=.3).
Kaplan-Meier curves for survival free from AF recurrence in patients with A) EATV stratified at 80mL; B) PATV stratified at 150mL; and C) attenuation of EAT posterior to LA stratified at -75 HU. The time axis starts after the blanking period (Time 0 corresponds to 3 months after the procedure). EATV, epicardial adipose tissue volume; HU, Hounsfield units; PATV, pericardial adipose tissue volume.
Uni- and multivariable Cox regression of adipose tissue measurements as dichotomous variable and as a continuous value indexed to BSA for AF recurrence
| AT measurement | No. of events /no. at risk | Univariable model | No. of events /no. at risk | Multivariable model * | ||
|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |||
| EATV ≥ 80 mL | 114/350 | 1.65 (1.14-2.39) | .007 | 102/317 | 1.24 (0.83-1.87) | .3 |
| EATVI | 90/280 | 1.02 (1.01-1.03) | .001 | 86/267 | 1.01 (0.99-1.02) | .4 |
| PATV ≥ 150 mL | 114/350 | 1.55 (1.07-2.24) | .019 | 102/317 | 1.10 (0.71-1.69) | .17 |
| PATVI | 90/280 | 1.01 (1.00-1.01) | .004 | 86/267 | 1.00 (1.00-1.01) | .6 |
| Attenuation EAT posterior to LA <-75 HU | 111/338 | 1.06 (0.73-1.56) | .7 | |||
95%CI, 95% confidence interval; AF, atrial fibrillation; EATV, epicardial adipose tissue volume; EATVI, epicardial adipose tissue volume indexed to body surface area; HR, hazard ratio; HU, Hounsfield units; LA, left atrium; PATV, pericardial adipose tissue volume; PATVI, pericardial adipose tissue volume indexed to body surface area.
We also assessed the association between EATV and total AF recurrence, including the blanking period. In this analysis, patients with EATV ≥ 80mL were also at higher risk of total recurrence (HR, 1.59; 95%CI, 1.12-2.27; P=.01)], but after multivariable adjustment, EATV ≥ 80mL did not persist as an independent predictor (HR, 1.17; 95%CI, 0.79-1.73; P=.4)] (detailed in ).
Similar results were observed after the indexation of EATV to total body surface area (table 2 and ).
Relationship between PAT volume and AF recurrenceWe found that patients with PATV ≥ 150mL had a higher risk of AF recurrence than patients with lower PATV (log-rank test P=.018), as shown in the Kaplan-Meir curves (figure 2B). This trend was observed in the Cox proportional hazards model, which showed that patients with PATV ≥ 150mL had a higher risk of recurrence (HR, 1.55; 95%CI, 1.07-2.24; P=.019], as detailed in table 2. After multivariable adjustment, PATV did not remain an independent predictor of AF recurrence (HR, 1.10; 95%CI, 0.71-1.69; P=.17).
In total recurrence analysis, patients with PATV ≥ 150mL were at higher risk of total recurrence (HR, 1.51; 95%CI, 1.06-2.15; P=.023), but after multivariate adjustment, PATV ≥ 150mL did not remain an independent predictor (HR, 1.03; 95%CI, 0.68-1.55; P=.9) ().
Similar results were observed after the indexation of PATV to body surface area (table 2 and ).
Association between attenuation of EAT posterior to the LA and AF recurrenceAssessment of the attenuation of EAT posterior to the LA was not possible in 12 patients (out of 350), as there was no tissue in range of−150 and−50 HU between the LA and the esophagus.
We did not observe a significant difference in AF recurrence between patients with attenuation of EAT posterior to the LA higher or lower than−75 HU (figure 2C, log-rank test P=.75). The same was observed in the univariable Cox proportional hazards regression (table 2, HR, 1.06; 95%CI, 0.73-1.56; P=.7).
Association between EAT volume and AF recurrence according to AF subtypeIn all multivariable Cox proportional hazards models, persistent AF was identified as the only independent predictor of AF recurrence (, all HR ranging from 1.89 to 2.19, and P-values below .011). Since AF subtype could have influenced our results, we performed an additional subanalysis to evaluate the effect of AF subtype on the primary outcome. In patients with paroxysmal AF, EATV ≥ 80mL was associated with an increased risk of recurrence, but not after adjustment to other covariates (HR, 1.31; 95%CI, 0.79-2.16; P=.3)], as shown in . For patients with persistent AF, no association was found for EATV ≥ 80mL and AF recurrence (HR, 0.92; 95%CI, 0.48-1.75; P=.8)].
DISCUSSIONThis is the largest study evaluating the relationship between various EAT parameters and AF recurrence after catheter ablation. We found that patients with an EATV ≥ 80mL had an increased risk of AF recurrence. Subgroup analyses according to the subtype of AF suggested that this association was only observed in patients with paroxysmal AF. However, after multivariable adjustment, EATV was not an independent predictor of AF recurrence, suggesting that this previously reported association was caused by confounding factors. In addition, the pattern of EAT attenuation posterior to the LA (which could be a marker of EAT inflammation) was not associated with AF recurrence in any analysis (figure 3).
Central illustration. Association between EAT parameters and the risk of AF recurrence. Of 350 patients included, 114 developed AF recurrence. Patients with an EATV ≥ 80mL had an increased risk of AF recurrence; however, after multivariable adjustment, EATV was not an independent predictor (HR, 1.24; 95%CI, 0.83-1.87; P=.3). The pattern of EAT attenuation posterior to the LA was not associated with AF recurrence. AF, atrial fibrillation; EAT, epicardial adipose tissue; EATV, epicardial adipose tissue volume; HU, Hounsfield units; IQR, interquartile range; LA, left atrium; PATV, pericardial adipose tissue volume.
Several studies have shown that obesity (evaluated by body mass index) is an independent risk factor for AF development.2 However, it is known that body mass index is an inaccurate way to evaluate the presence of excess adiposity4 and that heterogeneous distribution of adipose tissue largely explains different cardiometabolic risks seen in individuals with similar degrees of overall adiposity,3 due to distinct metabolic activity and proinflammatory mediators secreted by various fat depots.24 Previous studies have shown that EAT, a form of visceral adipose tissue, is correlated with cardiovascular disease risk factors,25 with an increased risk of coronary atherosclerotic burden5,6 and heart failure with preserved ejection fraction.9 Moreover, it is also known that EAT can induce cardiac remodeling, being associated with LA dilation, left ventricular hypertrophy, and diastolic dysfunction.7,8
Several pathophysiologic mechanisms might contribute to an ensuing association between EAT and AF. First, it is known that EAT is a local source of several proinflammatory adipokines (such as interleukin-1β and tumor necrosis factor-α), which can have a paracrine effect on myocardium and cardiac vessels.3,4,26 In addition, inflammation may promote atrial fibrosis and AF onset.26 The effect of EAT is facilitated by its proximity to cardiac structures, the absence of fascial barriers between fat and the myocardium, and by its shared blood supply.3,4,26 Moreover, inflammation could also impact the ablation procedure as edema could hamper proper ablation lesions formation.27 Secondly, several pathological studies have shown that EAT can infiltrate the myocardium and cause microe-entry circuits, possibly contributing to AF onset.3,26 Finally, autonomic dysfunction may also be involved, as the ganglionated plexus is located in epicardial fat and its inflammatory activation can cause shortening of action potentials.4,28
Although EAT was associated with AF recurrence in the univariable analysis, in our study, EAT was not an independent predictor of this outcome, suggesting the contribution of confounding factors. A Cox regression model including EAT plus each individual variable used for adjustment showed that dilated LA had the greatest impact. We also evaluated other parameters of EAT, such as PATV, showing similar findings. The first study that reported a possible association between EATV and AF recurrence, showed that large EATV was associated with postablation recurrence of AF (239.0±90.2cm3 vs 153.5±42.7cm3, P=.0002). Nevertheless, neither a recurrence-free survival nor a multivariable analysis was done to assess whether this was an independent predictor.29 There are other studies either supporting30,31 or contradicting32,33 these findings on EATV. Nakatani et al.30 did not find an association between EATV and AF recurrence but they evaluated a very small population (n=44). Maeda et al.32 showed that, in 218 Asian Japanese patients, EATV indexed to body surface area was a predictor of AF recurrence, independently of other risk factors (HR, 1.02; 95%CI, 1.00-1.03; P=.012). The discordance between our study and that by Maeda et al.32 might be explained by racial differences, since individuals from Asia have greater cardiovascular morbidity with lower amounts of overall adiposity and studies have shown that EAT effect estimates are stronger in these populations than in those from North America or Europe.3 Moreover, in our multivariable adjustment model, we included clinically relevant predictors of AF recurrence, whereas Maeda et al.32 only adjusted for variables that, on univariate analysis, had a P-value <.10.
The relationship between EAT and AF recurrence according to AF subtype (paroxysmal vs persistent AF)Our study suggests that EATV had a greater impact on AF recurrence in patients with paroxysmal vs persistent AF. Persistent AF is associated with more structural and electrical remodeling, which can impose a higher risk of recurrence.34 It is possible that, in paroxysmal AF cases, the proarrhythmogenic mechanisms of EATV could influence the risk of AF recurrence although this association might be mediated by confounding factors.
The impact of several EAT parameters and the risk of AF recurrenceThe measurement of EAT volume can be theoretically considered the most reliable parameter to measure epicardial fat.4 However, we chose to also evaluate the effect of other parameters (such as PATV and attenuation of EAT posterior to the LA as detailed in figure 1) on AF recurrence. It is known that, whereas EAT is within the pericardial sac, PAT corresponds to all fat in and outside the pericardium. PAT measurement does not require pericardium identification, allowing a quicker assessment, and does not require excellent image quality,4 which may contribute to an easier clinical implementation. Indeed, we found that effect estimates were smaller than EATV, in line with previous studies,3 since anatomical proximity to myocardium facilitates pathophysiological mechanisms in arrhythmogenesis.
Since inflammation of EAT could play a role in AF recurrence, and quality of adipose tissue, more than quantity, could be a more relevant predictor of AF recurrence, we also collected data on the attenuation of EAT measured posterior to the LA. It is known that inflammation shifts the attenuation of the adipose tissue toward a more positive value.17 Previous studies have associated the attenuation of EAT with culprit coronary lesions in acute coronary syndrome and cardiac mortality.15 Ciuffo et al.16 found that increased attenuation of periatrial adipose tissue measured from a single CT slice was a predictor of AF recurrence; whereas another study17 showed that increased attenuation of adipose tissue posterior to the LA, by volumetric assessment, was not a predictor after adjustment (HR, 1.26; 95%CI, 0.90-1.76; P=.181). In our study, we did not find any association between this parameter and the risk of AF recurrence. Therefore, our data support that attenuation of adipose tissue posterior to the LA is not useful in this setting, although EAT thickness and characteristics can vary across different myocardial regions.
Strengths and limitationsTo the best of our knowledge, this is one of the most detailed studies investigating the association between AF recurrence and various cardiac adipose tissue measurements and including a long-term follow-up. EAT was assessed mainly by calculating EATV, which requires individual and detailed pericardium manual tracing for each patient. The interclass correlation coefficient of this measurement was excellent. On multivariable analysis, we adjusted for several important clinical risk factors involved in AF recurrence to mitigate confounding.
There are some limitations that should be addressed. First, patient follow-up did not include continuous electric monitoring, via event or loop recorders, and therefore this study could not evaluate subclinical AF recurrence or AF burden. Second, this study excluded patients with coronary artery disease and valvular heart disease, and consequently, these conclusions cannot be extrapolated to these populations. Third, we did not evaluate if waist circumference, waist-to-hip ratio or CT-derived parameters of abdominal fat could have added more information on the effects of local vs overall adiposity.3,7
CONCLUSIONSEAT parameters including the evaluation of EAT volume, PAT volume and the attenuation of EAT are not independent predictors of AF recurrence after catheter ablation.
- –
Obesity is a known risk factor for AF development. However, body mass index is an inaccurate way to evaluate excess adiposity.
- –
Previous studies have shown that EAT, a form of visceral fat, is correlated with cardiovascular diseases.
- –
Few large studies have evaluated the influence of EAT on the risk of AF recurrence, focusing only on one method to quantify EAT.
- –
This is one of the largest and most detailed studies investigating the association between AF recurrence and various cardiac adipose tissue measurements.
- –
Both EATV and PATV were associated with AF recurrence in the univariable analysis; however, they did not remain independent predictors after adjustment for other clinical risk factors.
- –
The pattern of EAT attenuation posterior to the LA (a possible biomarker of local inflammation) was not associated with AF recurrence.
This study was financed by a) national funds through FCT Fundação para a Ciência e Tecnologia, I.P., under the scope of the Cardiovascular R&D Center – UnIC (UIDB/00051/2020 and UIDP/00051/2020) and b) European Regional Development Fund (ERDF), through the North Regional Operational Program in the framework of the project HEALTH-UNORTE: Setting-up biobanks and regenerative medicine strategies to boost research in cardiovascular, musculoskeletal, neurological, oncological, immunological and infectious diseases (NORTE-01-0145-FEDER-000039).
The sponsor was not involved in the study design, data collection, analysis or interpretation of the results, the writing of the manuscript, or the decision to publish.
AUTHORS’ CONTRIBUTIONSAll authors meet the following 4 criteria: a) contributed substantially to the conception and design, acquisition of data, or its analysis and interpretation; b) drafted the article or critically reviewed it; c) gave final approval to the version to be published; d) agreed to take responsibility for the accuracy and integrity of the manuscript. I. Cruz and S. Lopes Fernandes contributed equally to this article.
CONFLICTS OF INTERESTThe authors declare that they have no conflict of interest.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.11.006