Dual antiplatelet therapy (DAPT) duration after ST-segment elevation myocardial infarction (STEMI) remains a matter of debate.
MethodsWe analyzed the effect of DAPT on 5-year all-cause mortality, cardiovascular mortality, and cardiovascular readmission or mortality in a cohort of 1-year survivor STEMI patients.
ResultsA total of 3107 patients with the diagnosis of STEMI were included: 93% of them were discharged on DAPT, a therapy that persisted in 275 high-risk patients at 5 years. Cardiovascular mortality in patients on single antiplatelet therapy vs DAPT at 5 years was 1.4% vs 3.6% (P <.01), respectively, whereas noncardiovascular mortality was 3.3% vs 5.8% (P=.049) at 5 years. Cardiovascular readmission or mortality in patients with single antiplatelet therapy vs DAPT was 11.4% vs 46.5% (P <.001). Extended DAPT was independently associated with worse 5-year all-cause mortality (HR, 2.16; 95%CI, 1.40-3.33), cardiovascular mortality (HR, 2.83; 95%CI, 1.37-5.84), and cardiovascular readmission or mortality (HR, 5.20; 95%CI, 3.96-6.82). These findings were confirmed in propensity score matching and inverse probability weighting analyses.
ConclusionsOur results suggest the hypothesis that, in 1-year STEMI survivors, extending DAPT up to 5 years in high-risk patients does not improve their long-term prognosis.
Keywords
Platelet inhibition is essential for secondary prevention of atherothrombotic events.1 The number of agents and the length of treatment that provide sufficient protection against thrombotic events and allow low complication rates (mainly bleeding) remains under investigation. An acute coronary syndrome is commonly the result of plaque rupture and acute coronary obstruction of an epicardial coronary vessel due to exposure of thrombogenic material within the plaque, which generates platelet activation and adhesion and eventually thrombus formation.2 Platelet inhibition is even more important in cases of coronary artery stenting, which restores arterial flow by stretching the artery and implanting a metallic device, usually with a drug-eluting polymer, into the coronary vessel wall. Percutaneous coronary interventions (PCI) usually induce denudation of the intima, exposing thrombogenic material and triggering an inflammation cascade that eventually also promotes thrombosis.3
Studies performed early in the first generation of drug-eluting stents showed ischemic-prevention benefits for extended dual antiplatelet therapy (DAPT) beyond 12 months, mainly due to late and very late stent thrombosis events related to early drug-eluting stent technology.4 After more than 37 clinical trials with different inhibitors of platelet P2Y12 receptors, current antiplatelet treatment recommendations for patients surviving ST-elevation myocardial infarction (STEMI) include 12 months of aspirin combined with a P2Y12 receptor inhibitor.5
Recent ESC guidelines for non–ST-elevation myocardial infarction (2020) recommend individualizing the antiplatelet therapy strategy and the length of treatment, balancing the ischemic and bleeding risks during follow-up.6 In contrast, the STEMI guidelines were last updated in 2017.5 Since then, several studies and meta-analyses have observed similar cardiovascular prognosis with shorter DAPT treatment duration after PCI,7 and with a net benefit in patients with increased bleeding risk.8 Administering DAPT for shorter periods followed by single P2Y12 inhibitor treatment was shown to be noninferior to standard therapies.9,10 However, the cited studies merged patients with chronic and acute coronary syndromes, with different prognosis regarding presentation and associated clinical characteristics.11,12 Despite the potential benefits of shorter DAPT duration, extended DAPT has also promised potential benefits in terms of cardiovascular prognosis and prevention of recurrent thrombotic events.4,13 The consequences of extended use of DAPT are still under discussion because the trade-off between bleeding risk and the benefit of cardiovascular event prevention varies with age and other factors.14 Some studies suggest a lack of benefit15 while others report some improvements in long-term outcomes.4,16 Adequate and contemporary scores on optimal duration in STEMI are lacking.
The aim of the present work was to analyze the effect of antiplatelet treatment intensity (dual vs single) on the 5-year incidence of major events in 1-year survivor STEMI patients of the Atención hospitalaria del síndrome coronario (ATHOS) cohort.
METHODSParticipants with STEMI were selected from the ATHOS cohort of consecutive acute coronary syndrome patients. The aim of the ATHOS study was to determine interhospital variability in acute coronary syndrome management.17 We conducted a follow-up based on this retrospective cohort.
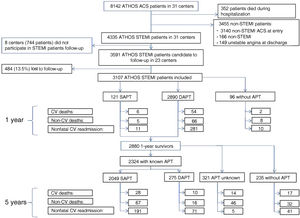
Of the 31 hospitals contributing data on 250 to 300 patients with acute coronary syndrome between 2014 and 2016, 23 participated in the long-term follow-up of the 3591 patients discharged with the diagnosis of STEMI. Antiplatelet therapy data were available for 86.5% of the 3107 1-year survivors and 2880 1-year survivors were further followed up at 5 years (figure 1). STEMI was defined according to current guidelines.5 Fibrinolysis was only considered if expected delays in primary PCI treatment were not acceptable and there were no contraindications. PCI postfibrinolysis was acutely performed in the absence of effective reperfusion and electively performed during the index admission.
Flow chart of patients recruited in the ATHOS cohort for 1- and 5-year follow-up and events observed. ATHOS (Atención hospitalaria del síndrome coronario; in English, In-hospital Treatment of Coronary Syndrome patients); ACS, acute coronary syndrome; APT, antiplatelet therapy; CV, cardiovascular; DAPT, dual antiplatelet therapy; MI, myocardial infarction; SAPT, single antiplatelet therapy; STEMI, ST-elevation myocardial infarction.
Baseline sociodemographic variables were recorded, as were variables on cardiovascular risk, treatment (including antiplatelet therapy), severity (in-hospital death, reinfarction, acute pulmonary edema, and cardiogenic shock), and major bleeding during admission. We included both ischemic and hemorrhagic strokes.
Vital status, readmissions for cardiovascular and other reasons, and antiplatelet therapy were obtained by personal examination, electronic medical records, or telephone interview in discharged patients at 1 year and again in 1-year survivors at 5 years.
We excluded from analysis 321 patients in whom antiplatelet therapy could not be ascertained between 1 and 5 years of follow-up, and 235 patients who could not receive antiplatelet therapy due to contraindications or other reasons. For those patients on DAPT at the time of an event of interest or at the end of the 5-year follow-up, we assumed that DAPT was never discontinued after the first year.
EndpointsPrimary endpoints, assessed at 5 years of follow-up, were cardiovascular mortality, mortality from noncardiovascular causes, and cardiovascular readmission or mortality.
Statistical methodsCategorical variables are presented as number and percentage, and continuous variables as mean and standard deviation or median and interquartile range, depending on their distribution. For categorical variable comparisons between DAPT and single antiplatelet therapy (SAPT), the chi-square test or Fisher exact test were used. For continuous variables, we used the Student t test or the Mann-Whitney U-test. Survival analysis was performed by Kaplan-Meier method and comparison between groups with the log-rank test.
The hazard ratio (HR) of the endpoints defined in ATHOS for DAPT was estimated by Cox proportional hazards models adjusted for confounding factors. Baseline characteristics were considered as potentially confounding if univariate analysis showed association at P-value <.10 with both DAPT and the variable of interest at follow-up (overall and cardiovascular mortality, and readmission or cardiovascular mortality). Models were also adjusted for clinically relevant variables such as age and sex. The proportionality requirements were analyzed through graphic visualization and through Grambsch and Therneau method.18
In addition, propensity score matching, and inverse propensity weighted analyses were performed. Propensity scores were computed as the estimated probability of receiving DAPT from a logistic regression including age, sex, smoking, hypertension, diabetes, previous myocardial infarction, previous stroke, previous peripheral artery disease, chronic obstructive pulmonary disease, kidney disease, acute pulmonary edema, or cardiogenic shock or Killip III-IV during admission. The latter was chosen over left ventricular ejection fraction due to the high correlation between the 2 variables and greater number of missing values in left ventricular ejection fraction (23.4%). In case of high correlation, we chose the variable with less missing data (eg, between diabetes and glycaemia, we selected diabetes).
For the propensity score matching, each SAPT patient were matched with 3 patients who received DAPT using a greedy nearest-neighbour matching algorithm without replacement within a range width of 0.2 standard deviations in the logit of propensity score. A proportional hazard Cox model with robust variance was applied and further adjusted for age and sex was fitted on the matched sample in the first approach and in the whole sample weighting for the inverse of propensity score in the second.
Ethics approval statementThis project was approved by the research ethics committee of IMIM-Hospital del Mar (reference 2014/5491/1) and declared NO-EPA by the Spanish Agencia del Medicamento y Productos Sanitarios (# 2258/ RG 4274). Procedures and data collection complied with the Declaration of Helsinki and Spanish Data Protection Laws. Patients were not informed of the present research because data were anonymously and retrospectively obtained from clinical registries by their attending physicians. Some patients (less than 20%) were recruited prospectively to obtain blood samples: all of them signed an informed consent.
Patient and public involvementThis project was initiated to spotlight the current differences in acute coronary syndrome management in Spain. None of the patients was involved in developing the research question, but the results of the study will be presented to the GICOR patient association to disseminate the conclusions of this study.
Transparency statementThis manuscript is an honest, accurate, and transparent account of the study being reported. No important aspects of the study have been omitted. Any discrepancies from the study as originally planned have been explained.
RESULTSThe 1-year follow-up was completed in 3107 (86.5%) of the 3591 patients with STEMI who were discharged alive from the 23 hospitals participating in the long-term follow-up of the ATHOS cohort, and 2880 1-year survivors were further followed up at 5 years (figure 1).
The baseline characteristics of 1-year survivors who then continued with DAPT (275) or SAPT (2049) differed in the previous history of cardiovascular diseases and cardiovascular procedures, which were more frequent in DAPT patients. Glomerular filtration rate and diabetes mellitus at admission were also significantly higher in DAPT patients (table 1).
Baseline (admission) characteristics of patients with ST-elevation myocardial infarction characteristics by SAPT or DAPT use between 1 and 5 years postdischarge.
| SAPT | DAPT | P | |
|---|---|---|---|
| n=2049 | n=275 | ||
| Type of acute coronary syndrome | .99 | ||
| ST-elevation acute coronary syndrome | 98.2 | 98.2 | |
| Nonclassifiable | 1.76 | 1.82 | |
| Age, y | 62.1±13.2 | 62.9±12.7 | .309 |
| Female sex | 20.2 | 17.5 | .321 |
| Smoking | 69.0 | 72.4 | .287 |
| Hypertension | 51.1 | 54.5 | .313 |
| Diabetes mellitus | 23.2 | 30.9 | .007 |
| Previous myocardial infarction | 9.81 | 24.7 | <.001 |
| Previous angina | 8.64 | 26.2 | <.001 |
| Previous stroke | 3.12 | 6.55 | .007 |
| Peripheral artery disease | 4.15 | 6.18 | .165 |
| Previous PCI | 8.20 | 26.2 | <.001 |
| Previous CABG | 1.07 | 6.55 | <.001 |
| Chronic respiratory disease | 7.96 | 9.45 | .461 |
| Chronic kidney disease | 4.49 | 5.45 | .573 |
| Glomerular filtration rate mL/min/m2 | 88.1±38.8 | 84.1±26.3 | .030 |
| Glycemia mg/dL | 149±64.6 | 160±82.2 | .040 |
| APE/CS or Killip III-IV | 6.83 | 9.82 | .094 |
| Left ventricular ejection fraction <30% | 20.1 | 22.7 | .416 |
| Major bleeding | 1.12 | 1.45 | .756 |
| Stroke during admission | 0.44 | 0.73 | .648 |
ACS, acute coronary syndrome; APE, acute pulmonary edema; CABG, coronary artery bypass graft; CS, cardiogenic shock; DAPT, dual antiplatelet therapy; MI, myocardial infarction; PCI, percutaneous coronary intervention; SAPT, single antiplatelet therapy.
The data are expressed as percentages of mean±standard deviation.
In addition to the differences in antiplatelet treatments that were to be expected in these patients, we observed a slight—but statistically significant—increase in use of statin, diuretics, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, and beta-blocker drugs in patients who continued DAPT beyond 1 year of follow-up (table 2).
Characteristics of patients with ST-elevation myocardial infarction by SAPT or DAPT use at 5 years postdischarge
| SAPT | DAPT | P | |
|---|---|---|---|
| n=2049 | n=275 | ||
| Atrial fibrillation | 3.65 | 3.30 | .903 |
| Treatments | |||
| Automatic implantable defibrillator | 0.34 | 0.73 | .289 |
| Pacemaker | 0.39 | 0.00 | .607 |
| Statin | 91.3 | 96.7 | .003 |
| ACE-inhibitors or ARB | 70.5 | 80.6 | .001 |
| Diuretics | 23.6 | 28.3 | .100 |
| Beta-blockers | 77.3 | 84.7 | .007 |
| Aspirin | 95.7 | 100 | .001 |
| Clopidogrel | 3.90 | 67.6 | <.001 |
| Ticlopidine | 0.00 | 0.73 | .014 |
| Prasugrel | 0.15 | 14.2 | <.001 |
| Ticagrelor | 0.29 | 18.5 | <.001 |
| Any P2Y12inhibitor | 4.32 | 100 | <.001 |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blockers; DAPT, dual antiplatelet therapy; SAPT, single antiplatelet therapy.
The data are expressed as percentages.
Figure 1 details the types of events that occurred in SAPT and DAPT patients at 5 years.
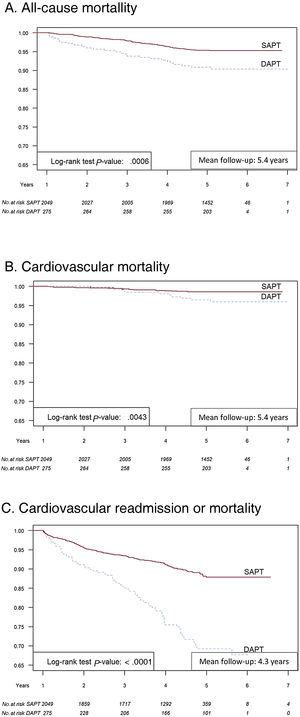
Figure 2 presents Kaplan-Meier event-free survival at 5 years for all-cause mortality (figure 2A), cardiovascular mortality (figure 2B), and cardiovascular readmission or mortality (figure 2C) in SAPT and extended DAPT patients. DAPT patients had significantly worse prognosis at 5 years than SAPT patients for all the endpoints.
Central illustration. Kaplan-Meier event-free survival at 1 and 5 years. All-cause mortality (A), cardiovascular mortality (B), and cardiovascular readmission or mortality (C) in single antiplatelet therapy and extended dual antiplatelet therapy patients. DAPT, dual antiplatelet therapy; SAPT, single antiplatelet therapy.
We then adjusted the effect of DAPT for potential confounders: variables significantly associated with 5-year events () and with the type of antiplatelet therapy (table 1 and table 2). Extending DAPT beyond 1 year was associated with worse overall mortality, cardiovascular mortality, and the combined endpoint of cardiovascular readmission or mortality even when adjusting for confounders (table 3). Propensity score matching 1:3, and inverse probability weighted proportional hazards models () confirmed these findings.
Hazard ratio of overall mortality, cardiovascular mortality, and cardiovascular readmission or mortality for dual compared with single antiplatelet therapy at 5 years after admission for an ST-elevation myocardial infarction
| Nonadjusted | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| 1- to 5-year follow-up | ||||
| Overall mortality | 2.10 (1.36-3.24) | 2.16 (1.40-3.33) | 1.79 (1.14-2.87) | 1.64 (1.04-2.58) |
| CV mortality | 2.74 (1.33-5.65) | 2.83 (1.37-5.84) | 2.32 (1.11-4.87) | 2.12 (1.00-4.49) |
| CV mortality or readmission* | 2.82 (2.15-3.69) | 2.78 (2.12-3.64) | 2.5 (1.9-3.29) | 2.46 (1.87-3.24) |
95%CI, 95% confidence interval; CV, cardiovascular; HR, hazard ratio.
Model 1: Age+female sex.
Model 2: Model 1+smoking+hypertension+diabetes+previous myocardial infarction+previous stroke+previous peripheral artery disease+chronic obstructive pulmonary disease+kidney disease.
Model 3: Model 2+acute pulmonary edema or cardiogenic shock or Killip III-IV on admission.
In this real-world retrospective cohort of patients with STEMI, in 1-year survivors who were continued on DAPT, overall mortality, cardiovascular mortality, and cardiovascular readmission was increased at 5 years, compared with SAPT.
Post-STEMI DAPT is recommended in current guidelines5 up to 12 months, essentially based on the expectation of reducing stent thrombosis and progression to future ischemic events not related to the index coronary lesion TRITON-TIMI 3819 and PLATO studies.20 Both studies randomized patients between 2004 and 2008; with 26% and 38% STEMI, respectively. More recently, a substudy of the PEGASUS-TIMI 54 trial,16 in which approximately 55% of patients were admitted for STEMI, showed that reinitiating DAPT more than 12 months after STEMI conferred no benefit in 3-year cardiovascular death, stroke, or myocardial infarction compared with placebo; however, it increased major bleeding. Similarly, in the ATHOS cohort, patients on DAPT had better all-cause and cardiovascular event-free survival, although both were restricted to the first year. Thereafter, and up to 5 years, maintaining DAPT was associated with a worse prognosis for all endpoints.
Long-term DAPT after STEMI has received scant attention in randomized clinical trials. The DAPT trial4 included 9961 patients treated with first- and second-generation drug-eluting stents, with only 10% being STEMI. Complications driven by stent thrombosis, myocardial infarction, and noncardiovascular death favored 30-month DAPT despite higher rates of major bleeding at 33 months of follow-up. After this recommendation, the proportion of patients with STEMI receiving extended DAPT for more than 12 months increased from 51% in 2010 to 77% in 2015 in Catalonia, Spain.21 In the 20-country EPICOR study, 49% of patients with STEMI were on DAPT at 2 years of follow-up.22 Those with longer DAPT duration were older, had more diabetes mellitus, and were more usually treated with medical therapy.
The DAPT substudy published in 201623 issued a prediction score for ischemic events with a modest capacity to discern the need for 12 months of DAPT vs 18 months. This score included the presence of diabetes mellitus, stent smaller than 3mm, smoking, first-generation drug-eluting stents, heart failure, low ejection fraction, saphenous vein grafting, and presentation as myocardial infarction, prior myocardial infarction, or PCI. STEMI patients with long-term DAPT in the ATHOS cohort, mainly with aspirin and clopidogrel, more frequently had a previous cardiovascular event and/or diabetes mellitus. However, extended DAPT was not associated with a better overall prognosis even when adjusted for clinical features of high residual ischemic risk.
The supposed benefit of extended DAPT in ATHOS may not outweigh a worse baseline characteristic of poor prognosis. Ischemic residual risk after a myocardial infarction is thought to be equally attributable to culprit and nonculprit lesions.24 However, once the stent thrombosis rate declines to less than 1% per year,24,25 the prevailing residual ischemic risk is associated with nonculprit lesions, which can be better evaluated and treated with aggressive lipid-lowering strategies.
In the Optidual trial,26 with only 12% of patients having STEMI, extending DAPT up to 22 months did not achieve statistical superiority in any endpoints. In the Smart-Date trial,27 extending DAPT at a median of 17 months did not decrease the primary endpoint but decreased the rate of myocardial infarction, accompanied by a tendency to increase bleeding rates. In the ATHOS cohort, the causes of 1-year readmission did not differ between the SAPT and DAPT groups. At 5 years, patients with DAPT were more frequently admitted for myocardial infarction and other cardiovascular diseases ().
The only randomized study in patients with STEMI, DAPT-STEMI,28 showed that 6 months of DAPT was noninferior in terms of complications, compared with the standard regimen of 12 months. The novelty of this study was that two-thirds of patients were treated with ticagrelor or prasugrel, which may confer acute ischemic protection during the first months after the index admission but may also tend to increase bleeding rates during the following months. Only a third of patients in the ATHOS cohort received DAPT with prasugrel or ticagrelor at discharge and follow-up.
LimitationsThis is a long-term follow-up of consecutive patients with STEMI, most of whom were treated with PCI. We were able to follow 71% of the original ATHOS patients with STEMI, as 23 of 31 participating centers engaged in the full 5-year follow-up. The baseline characteristics of participants and nonparticipants were quite similar (), which indicates that little bias, if any, exists in our results.
At the time the study was initiated, there was no validated DAPT score published to predict thrombotic or bleeding events. So far, there is still limited evidence to make precise adjustments in long-term antithrombotic treatment in STEMI patients.
Study limitations included the loss of 13.6% of candidate patients; nonetheless, differences in the baseline characteristics of participants and those lost to follow-up were nonsignificant or clinically irrelevant (). The number of patients on DAPT after 1 year was 275 compared with more than 2000 on SAPT: we assumed that all of them continued treated with DAPT up to an endpoint or to the end of follow-up. However, given that no clinical trial randomizing DAPT to such a long-term is available, we deem our message particularly important.
There were no available data on the need of anticoagulation treatment in this cohort at discharge following the index admission; however, the rate of atrial fibrillation, one of the major indications for anticoagulation, was similar between the SAPT and DAPT groups. Bleeding during follow-up is an important issue,29 and was not ascertained. Presumably, severe bleeding could have caused a readmission or mortality that might have been included in our combined endpoint of mortality or cardiovascular readmission; nonetheless, this lack of data is a limitation. The DAPT or SAPT were assessed at the time of an endpoint or at the end of follow-up and assumed to have been continued unchanged since the beginning of follow-up, 1 year after the index STEMI. Finally, the results of our retrospective cohort design may reflect differences in the prognosis of patients with different inherent unmeasured risks; this also shows the difficulty of correctly assessing and treating long-term cardiovascular risk after a coronary artery ischemic event.
CONCLUSIONSOur results suggest the hypothesis that, in 1-year STEMI survivors, extending DAPT up to 5 years in high-risk patients does not improve their long-term prognosis.
- –
STEMI patients have a prognostic benefit with DAPT up to 1 year. Evidence of the benefit of extending DAPT beyond 1 year is scarce in STEMI setting.
- –
In a contemporary cohort of STEMI patients, we found that extending DAPT beyond 1 year in high-risk STEMI patients does not seem to improve their long-term prognosis.
This study has received the following funding: CIBERCV for research in cardiovascular diseases; European Regional Development Funds - ERDF; FISP12/03287; FIS-CP12/03287, FIS-14/00449, FIS-PI081327, FIS-INTRASALUDPI18/00030; Agència de Gestió d’Ajuts Universitaris de Recerca (AGAUR) de Catalunya, 2017SGR222.
AUTHORS’ CONTRIBUTIONSConception of the study: J. Marrugat, I. Subirana, F. Fernández-Avilés, P.L. Sánchez, M. Roqué, D. Fernández-Bergés, J. Sanchis, and R. Elosua. Study data acquisition: all authors. Data analysis: A. Toloba, H. Tizón-Marcos, and I. Subirana. Interpretation of results and preparation of the manuscript: H. Tizón -Marcos, R. Elosua, and J. Marrugat. All authors are responsible for critically reviewing the manuscript and for approving its final version. Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki.
CONFLICTS OF INTERESTJ. Sanchis is editor-in-chief of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed.
The rest of the authors declare no conflict of interest.
The authors are grateful to the full roster of ATHOS study investigators and collaborators, which can be found in the supplementary data, for data acquisition.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.12.003