Intraventricular thrombosis is a dreaded complication of myocardial infarction, and its prevalence remains high despite early revascularization and significant improvements to antithrombotic treatments in recent decades.1 The estimated incidence of intraventricular thrombosis after myocardial infarction ranges from 3% to 10%, depending on the imaging modality used.2,3 The cumulative incidence of ischemic stroke in the first 2 years after a myocardial infarction is approximately 3% to 5%.4 In the absence of atrial fibrillation, most postinfarction ischemic strokes are attributed to an intraventricular thrombus. The predominant hypothesis on the pathophysiology of postinfarction thrombi involves 3 interacting factors: a) a systemic proinflammatory/procoagulant state; b) endocardial damage with subsequent platelet aggregation and activation; and c) blood stasis resulting in akinetic and dyskinetic myocardial regions that disrupt normal blood flow within the ventricular cavity.5
Early and accurate diagnosis of intraventricular thrombosis following myocardial infarction directly affects both treatment and clinical outcomes. Cardiac magnetic resonance imaging (MRI) is the preferred diagnostic tool this condition.6 While transthoracic echocardiography (TTE) is the preferred option for evaluating biventricular function and mechanical complications after myocardial infarction, it has poor sensitivity for detecting thrombi. Compared with cardiac MRI, its detection rate is approximately 30%, but can be as high as 64% with contrast agent use.2 Cardiac MRI primarily outperforms TTE at detecting intraventricular thrombi because of its tissue characterization capabilities. Using perfusion sequences and late gadolinium enhancement, cardiac MRI can reveal a lack of vascularization, easily distinguishing thrombi from the surrounding myocardium.5 The use of cardiac MRI after myocardial infarction, however, is limited by its restricted clinical availability and long exam times, which are often associated with patient discomfort and claustrophobia (a complete cardiac evaluation with conventional sequences can take approximately 45minutes).
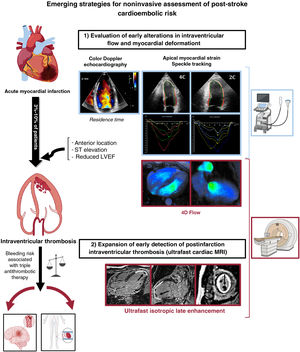
These limitations have prompted the search for techniques that could improve the likelihood of identifying patients at risk of cardioembolic stroke after myocardial infarction. One approach is the use of imaging techniques to map and quantify intraventricular blood stasis. In a recent article published in Revista Española de Cardiología, Rodríguez-González et al.7 prospectively analyzed the ability of intraventricular blood stasis imaging with TTE to predict cardioembolic events after ST-segment elevation myocardial infarction (STEMI). The single-center ISBITAMI trial prospectively enrolled patients with a first STEMI, a left ventricular ejection fraction (LVEF) ≤45%, and no history of significant carotid artery or valve disease, conditions that could potentially cause neurological damage, contraindications for MRI, atrial fibrillation, or other indications for long-term anticoagulation therapy. On inclusion, patients underwent speckle tracking TTE with assessment of blood stasis and apical longitudinal strain in the left ventricle. Cardiac and cerebral MRI was performed 1 week and 6 months after STEMI. The primary composite endpoint included the occurrence of intraventricular thrombosis, transient ischemic attack or ischemic stroke, acute or subacute silent cerebral infarction, and/or peripheral systemic embolism from inclusion to the 6-month follow-up visit.
Of the 92 patients initially included, 75 underwent cardiac MRI at 1 week. After excluding losses to follow-up and deaths due to other causes, the authors included 66 patients (78% male; median age, 58 years) in the final analysis. The patients predominantly had anterior infarct location (89%) and a median LVEF of 41%, as determined by TTE. Seventeen patients (25%) experienced at least 1 event in the primary composite endpoint. While this incidence is high compared with other cohorts,4 13 of the 17 events were intraventricular thrombi. There were only 5 silent cerebral infarctions and 3 strokes or transient ischemic attacks. No systemic embolisms were observed in other regions. Supporting previous reports,1 all the events except 1 silent cerebral infarction were detected in the cardiac or cerebral MRI studies performed at 1 week. There were no differences in baseline clinical or laboratory characteristics between patients who had experienced an event and those who had not. Nonetheless, baseline TTE showed that all the patients with an event had an anterior STEMI location. They also had a significantly lower median LVEF, worse apical longitudinal strain, and longer residence times (an indicator of intraventricular stasis). In the multivariable regression analysis including LVEF, residence time, and apical strain, only residence time and apical strain were significantly associated with the primary composite endpoint. Each parameter had a better C statistic than LVEF in both the TTE and cardiac MRI studies, indicating that they were better at identifying patients at risk of intraventricular thrombosis and cardioembolic events after STEMI. Their predictive ability was even greater when used in combination.
The work by Rodríguez-González et al.7 is particularly pertinent, as the authors have evaluated a much-needed clinical tool that could potentially facilitate complex clinical decisions, such as whether to start a patient on prophylactic anticoagulant therapy following myocardial infarction, with due consideration of the bleeding risks associated with triple therapy. The 2023 European guidelines do not recommend specific preventive strategies in this regard but do emphasize the importance of personalized monitoring with imaging studies and highlight the need for more randomized clinical trials to address gaps in the current evidence.8 The study by Rodríguez-González et al. also has some methodological limitations, including its small sample size, considerable loss to follow-up, the use of conventional functional parameters (LVEF) of limited value for studying the source of embolisms for comparison purposes, and the use of a primary endpoint comprising clinical and subclinical cardioembolic events and intraventricular thrombosis itself. The authors provide a balanced interpretation of their results and recognize that their study is a proof-of-concept study and that their findings require validation.
From a technical perspective, Rodríguez-González et al.7 evaluated blood stasis by calculating the residence time of a volume of blood within the myocardial cavity through velocity measurements obtained from the segmented left ventricular cavity using 2-dimensional Doppler TTE. For their estimates, they used a Lagrangian approach, assuming zero diffusivity of the blood relative to its movement with each heartbeat. The study was prompted by earlier preclinical work by their group showing that consideration of residence time improved predictions of cerebral microemboli after acute myocardial infarction.9 Given the limited clinical evidence on the optimal duration of anticoagulant treatment, an additional question is whether a better understanding of changes in blood stasis patterns during follow-up might guide personalized decisions on optimal treatment durations. The longitudinal design of the ISBITAMI study, for instance, could have been leveraged to determine different trajectories of stasis patterns. It should also be noted that the predictive value of residence time remains to be compared with that of other validated intracardiac flow parameters, such as cardiac MRI 4-dimensional flow parameters, which include 3-dimensional information.10
As part of a broader strategy aimed at improving the prediction of cardioembolic events in patients who have experienced myocardial infarction, an alternative to the early evaluation of flow dynamics as proposed by Rodríguez-González et al.7 would be to expand the use of cardiac MRI to diagnose intraventricular thrombosis in the early postinfarction period (figure 1). Ultrafast MRI sequences, such as ESSOS (enhanced sensitivity encoding [SENSE] by static outer volume subtraction), allow for a complete cardiac study (biventricular function and ultrafast isotropic late enhancement) in just 2 breath-holds (40seconds), overcoming most of the previously mentioned limitations of conventional cardiac MRI. Although ESSOS cardiac MRI has been clinically validated in a range of cardiovascular settings,11 its potential to modify treatment strategies and ultimately improve prognosis after a myocardial infarction has yet to be demonstrated.
Emerging strategies for the noninvasive assessment of postinfarction cardioembolic risk. LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging. Cardiac MRI 4-dimensional flow image courtesy of Dr Álvarez-Vázquez, Hospital Universitario Quirónsalud, Madrid. Figure created with the aid of BioRender (https://biorender.com/).
In conclusion, the work of Rodríguez-González et al.7 supports the value of combining parameters (myocardial strain and intraventricular stasis [TTE]) to improve the prediction of cardioembolic complications following myocardial infarction. One of the most interesting aspects of the study, which opens up new lines of research, is the potential use of blood stasis imaging in other clinical conditions, such as left atrial stasis and ventricular stasis in nonischemic dilated cardiomyopathy in patients with reduced LVEF but no other indications for anticoagulation therapy. The authors also highlight opportunities for clinical trials to test the efficacy of stasis imaging in guiding prophylactic oral anticoagulant therapy in selected patients. This is welcome news in a field that needs more robust contemporary evidence.
FUNDINGThis study received no funding. The Centro Nacional de Investigaciones Cardiovasculares Carlos III is supported by the Instituto de Salud Carlos III, the Spanish Ministry of Science and Innovation, and the ProCNIC Foundation. It is a Severo Ochoa center of excellence (CEX2020-001041-S funded by MICIN/AEI/10.13039/501100011033).
CONFLICTS OF INTERESTNone.