The shortage of donor hearts, lengthening [0]waiting times, and the need for urgent transplants1 necessitate an expansion of the clinical criteria for heart transplant (HT) to include donors with a nonstandard-risk. This category includes patients on veno-venous extracorporeal membrane oxygenation (V-V ECMO) as bridge therapy to lung transplantation or recovery. Not all of these patients survive, creating a niche of potential donors. We present the case of an adult male patient who underwent successful HT from a brain-dead donor on V-V ECMO.
The donor was a 28-year-old man with labial herpes simplex virus infection who was admitted to hospital with fever, myalgia, and cough. A chest X-ray and computed tomography scan indicated bilateral pneumonia. Blood cultures were negative, and serological analysis detected immunoglobulin M (IgM) antibodies to syncytial respiratory virus. The patient was placed on broad-spectrum antibiotic therapy and required mechanical ventilation. A tracheostomy was performed on day 14 of hospitalization, and the patient was transferred to our hospital. After hospitalization for 19 days, the patient's worsening respiratory profile required respiratory V-V ECMO support (Cardiohelp-Getinge Group, Sweden) with cannulation of the right femoral and jugular veins. The patient was treated according to the local protocol, including continuous anticoagulation with sodium heparin to maintain the activated coagulation time between 160 s and 180 s. On post-HT day 9, the patient developed bilaterally fixed and dilated pupils, and cranial computed tomography detected evidence of hypoxic-ischemic encephalopathy. Brain death was confirmed by clinical examination and cerebral computed tomography angiography. An apnea test was inconclusive for hypoxemia.
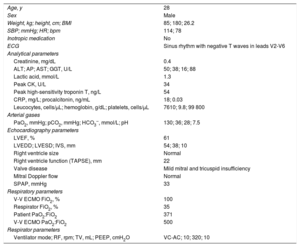
After obtaining family consent, the patient was painstakingly evaluated as a potential donor. Favorable results were obtained from hemodynamic and respiratory analysis and ancillary tests (table 1). The heart, liver, and kidneys were removed under V-V ECMO support.
Donor clinical and hemodynamic characteristics and ancillary test results
| Age, y | 28 |
| Sex | Male |
| Weight, kg; height, cm; BMI | 85; 180; 26.2 |
| SBP; mmHg; HR; bpm | 114; 78 |
| Inotropic medication | No |
| ECG | Sinus rhythm with negative T waves in leads V2-V6 |
| Analytical parameters | |
| Creatinine, mg/dL | 0.4 |
| ALT; AP; AST; GGT, U/L | 50; 38; 16; 88 |
| Lactic acid, mmol/L | 1.3 |
| Peak CK, U/L | 34 |
| Peak high-sensitivity troponin T, ng/L | 54 |
| CRP, mg/L; procalcitonin, ng/mL | 18; 0.03 |
| Leucocytes, cells/μL; hemoglobin, g/dL; platelets, cells/μL | 7610; 9.8; 99 800 |
| Arterial gases | |
| PaO2, mmHg; pCO2, mmHg; HCO3–, mmol/L; pH | 130; 36; 28; 7.5 |
| Echocardiography parameters | |
| LVEF, % | 61 |
| LVEDD; LVESD; IVS, mm | 54; 38; 10 |
| Right ventricle size | Normal |
| Right ventricle function (TAPSE), mm | 22 |
| Valve disease | Mild mitral and tricuspid insufficiency |
| Mitral Doppler flow | Normal |
| SPAP, mmHg | 33 |
| Respiratory parameters | |
| V-V ECMO FiO2, % | 100 |
| Respirator FiO2, % | 35 |
| Patient PaO2:FiO2 | 371 |
| V-V ECMO PaO2:FiO2 | 500 |
| Respirator parameters | |
| Ventilator mode; RF, rpm; TV, mL; PEEP, cmH2O | VC-AC; 10; 320; 10 |
AF, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CK, total creatine kinase; CRP, C-reactive protein; ECG, electrocardiogram; FiO2, fraction of inspired oxygen; GGT, gamma-glutamyl transpeptidase; HCO3–, bicarbonate; HR, heart rate; IVS, interventricular septum; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; V-V ECMO, veno-venous extracorporeal membrane oxygenation; PaCO2, partial pressure of arterial carbon dioxide; PaO2: partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure; RF, respiratory frequency; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TV, tidal volume; VC-AC, volume control-assist control.
The recipient was a 55-year-old man (87 kg, 167 cm) diagnosed with hypertrophic cardiomyopathy and with a previous sternotomy. He had been on the elective HT waiting list for 75 days.
Orthotropic HT was performed by the bacaval method with standard sternotomy. Total ischemia time was 125 min, and the patient showed good postoperative progress. Post-transplant echocardiography detected good biventricular function of the donor organ. The patient was discharged 17 days after admission under treatment with standard doses of mycophenolate mofetil, tacrolimus, and prednisone. Over 48 weeks of follow-up, the patient underwent 6 scheduled endomyocardial biopsies: 4 showing mild cellular rejection (grade 1R on the ISHLT 2004 working formulation) and 2 showing no evidence of rejection. The patient developed febrile neutropenia in post-HT week 11, with no signs of infection.
The use of V-V ECMO has increased due to the accumulated experience with this procedure and technological advances. The indications for V-V ECMO have expanded as a result, and this approach has become more generally used at more hospitals. The overall incidence of complications is 7.1%, with brain death accounting for 23% of them.2
Published data appear to confirm that organs from brain-dead donors supported by V-V or veno-arterial ECMO perform similarly to those from brain-dead donors without ECMO support.3-5 Despite the evidence for the safety of organs transplanted from ECMO-supported donors,4 there is a continuing reluctance among transplantation teams to use organs from these patients, owing to the difficulty of the apnea test, the frequency of concomitant multiorgan failure, and the lack of information about associated pathophysiological changes.
Patients on V-V ECMO support eventually undergo an increase in lung pressure secondary to hypoxia, and the resulting increase in right ventricular load can affect the systo-diastolic function of the grafted organ.6 Appropriate flow rates should be maintained through the ECMO cannulas to guarantee adequate organ perfusion.6 Transplant teams should also carry out an exhaustive assessment of the donor heart, confirm stable respiratory parameters and the absence of multiorgan failure, and consider other factors related to the success of HT, such as donor-recipient age match and a short predicted ischemia time to protect the grafted heart from the inflammatory response to ischemia-perfusion.
The clinical and radiological characteristics of our donor are consistent with COVID-19; however, the clinical case occurred in December 2019, before the identification of COVID-19 in Spain or the use of SARS-CoV-2 PCR tests. COVID-19 was later excluded by testing serum samples for antibodies (IgM and IgG). The inverted T waves in the patient's electrocardiogram were attributed to brain damage.
There are few reports in the literature on the therapeutic transplantation of donor hearts from patients on V-V ECMO support, and there is no standardized protocol for this procedure. The case reported here is the first in Spain unaffected by problems related to graft function, the number and severity of rejections, or complications due to infection. However, it is important to proceed with caution, since this was the case of a single transplant recipient with a short follow-up, and there is little literature on this topic.