Reducing low-density lipoprotein cholesterol (LDLc) with lipid-lowering therapy decreases cardiovascular events in both primary and secondary prevention; hence, the sharper the decrease, the lower the cardiovascular risk and the earlier the decrease occurs.1 Despite receiving statin therapy alone or in combination with ezetimibe, only 25% to 30% of patients in Spain with ischemic heart disease achieve the recommended LDLc targets.2
Proprotein convertase subtilisin/kexin type 9 inhibitors are highly effective in lowering LDLc levels and the risk of cardiovascular complications. In the FOURIER3 study, patients with established atherosclerotic cardiovascular disease showed significant reductions in cardiovascular events when evolocumab was added to the standard lipid-lowering therapy. However, as there may be substantial differences between clinical trials and “real life”, it is essential to know how these drugs perform in clinical practice. Unfortunately, there are very few studies analyzing the role of evolocumab in real-life settings4,5 and those that exist include only a small number of patients.
The RETOSS-CARDIO study (RETrospective Observational Study of Evolocumab Use in Spanish Cardiology Units), endorsed by the Spanish Research Agency of the Spanish Society of Cardiology, was designed to analyze the effect evolocumab on the lipid profile and its safety in the real world of patients treated in hospital cardiology units in Spain. This retrospective, multicenter, observational study analyzed the medical records of patients starting evolocumab in the usual clinical practice of Spanish hospital cardiology units between February 2016 and May 2017 (first years after publication of the therapeutic positioning report). The study was approved by the Ethics Committee of University Hospital La Paz in Madrid. Data were collected retrospectively from the 12 weeks before the start of treatment until 12 weeks after treatment initiation.
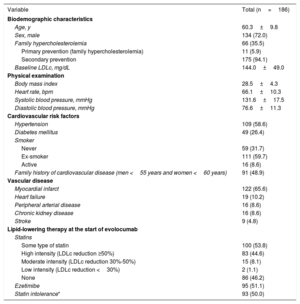
In total, 186 patients were included: mean age was 60.3±9.8 years, 35.5% had a family history of hypercholesterolemia, 94.1% had experienced a previous cardiovascular event, and baseline LDLc was 144.0±49.0mg/dL. At baseline, 53.8% were taking statins (high intensity in 44.6%, moderate intensity in 8.1%, and low intensity in 1.1%) and 51.1% were taking ezetimibe. Among the total, 50% had total or partial statin intolerance (table 1).
Patients’ baseline characteristics
| Variable | Total (n=186) |
|---|---|
| Biodemographic characteristics | |
| Age, y | 60.3±9.8 |
| Sex, male | 134 (72.0) |
| Family hypercholesterolemia | 66 (35.5) |
| Primary prevention (family hypercholesterolemia) | 11 (5.9) |
| Secondary prevention | 175 (94.1) |
| Baseline LDLc, mg/dL | 144.0±49.0 |
| Physical examination | |
| Body mass index | 28.5±4.3 |
| Heart rate, bpm | 66.1±10.3 |
| Systolic blood pressure, mmHg | 131.6±17.5 |
| Diastolic blood pressure, mmHg | 76.6±11.3 |
| Cardiovascular risk factors | |
| Hypertension | 109 (58.6) |
| Diabetes mellitus | 49 (26.4) |
| Smoker | |
| Never | 59 (31.7) |
| Ex-smoker | 111 (59.7) |
| Active | 16 (8.6) |
| Family history of cardiovascular disease (men <55 years and women <60 years) | 91 (48.9) |
| Vascular disease | |
| Myocardial infarct | 122 (65.6) |
| Heart failure | 19 (10.2) |
| Peripheral arterial disease | 16 (8.6) |
| Chronic kidney disease | 16 (8.6) |
| Stroke | 9 (4.8) |
| Lipid-lowering therapy at the start of evolocumab | |
| Statins | |
| Some type of statin | 100 (53.8) |
| High intensity (LDLc reduction ≥50%) | 83 (44.6) |
| Moderate intensity (LDLc reduction 30%-50%) | 15 (8.1) |
| Low intensity (LDLc reduction <30%) | 2 (1.1) |
| None | 86 (46.2) |
| Ezetimibe | 95 (51.1) |
| Statin intolerance* | 93 (50.0) |
HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol.
In all cases, patients were prescribed an initial evolocumab dose of 140mg, mainly every 2 weeks (97.3%). The treatment was interrupted in only 6 patients (3.2%): in 5 cases at the patient's request (with no mention of adverse effects) and in 1 case due to myalgia (0.5%), although causality was not conclusively demonstrated. Treatment adherence was adequate in most patients (92.3%).
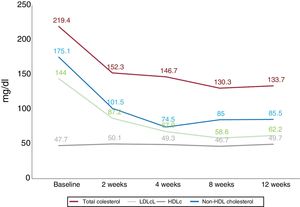
Evolocumab therapy was associated with significant reductions in total cholesterol, LDLc, and triglycerides. Levels of high-density lipoprotein cholesterol did not significantly change during follow-up (figure 1). At 12 weeks, 82.5% of patients had LDLc<100mg/dL, 64.9%, <70mg/dL, and 49.1%, <50mg/dL.
This study analyzed a relatively large population of patients who received evolocumab for the first time in Spanish cardiology units in accordance with clinical practice and the therapeutic positioning report. The main results show that evolocumab led to marked LDLc reductions with virtually no adverse effects, and that LDLc targets were achieved in a high percentage of patients. One recent study performed in the Unites States in patients with atherosclerotic cardiovascular disease found that only 15% of patients in clinical practice met the inclusion/exclusion criteria of the FOURIER6 study, indicating that real-life studies such as ours are needed to complete the information from clinical trials.
We found that evolocumab administration was associated with significant LDLc reductions starting from day 2 of treatment. These decreases were maintained and even increased over the following weeks of treatment to reach almost 60% at 12 weeks, which led to high percentages of LDLc control during follow-up. These findings concur with those observed in the FOURIER study.3
Adherence to therapy was very high (>92%), and the regimen was interrupted in only 1 patient (0.5%) due to adverse effects. Tolerance to evolocumab was very good in both the clinical trials and clinical practice studies, with very low interruption rates.3–5
In conclusion, the RETOSS-CARDIO study is the first national registry of patients treated with evolocumab in hospital cardiology units in Spain. Evolocumab therapy was associated with LDLc reductions close to 60% at 12 weeks, with good adherence and very low discontinuation rates due to adverse events. These data, obtained in dyslipidemia patients in Spanish cardiology units, are consistent with those reported in the FOURIER study.
FUNDINGThis study was sponsored by Amgen España, which had no influence on its development, and was validated by the Research Agency of the Spanish Society of Cardiology.
CONFLICTS OF INTERESTC. Roldan works in the Amgen Medical Department. The remaining authors have received fees from Amgen for consulting/presentations.