Transcatheter aortic valve replacement has become the treatment of choice for inoperable and high-risk patients with symptomatic aortic stenosis, and is becoming more and more common as the first choice for intermediate-risk patients as well. The next step in this evolution would be the expansion of treatment indications to low-risk patients. Successful treatment of this patient population will require setting new standards in terms of clinical outcomes and cost effectiveness. In this review, we present the main challenges that need to be addressed before transcatheter aortic valve replacement can be applied as a standard treatment for low-risk patients.
Keywords
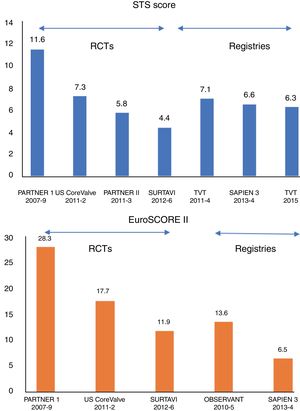
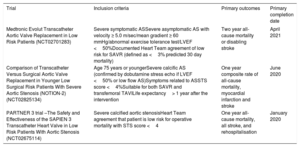
In less than a decade since publication of the PARTNER 1 trial, transcatheter aortic valve implantation (TAVI) has revolutionized the treatment of aortic stenosis (AS). TAVI is now the treatment of choice for inoperable and high surgical risk patients,1 and strong evidence support its use over surgical aortic valve replacement (SAVR) in intermediate-risk patients.2,3 Indeed, registry data show that the average surgical risk of TAVI patients in real-world practice is already in the intermediate range4 (Figure 1). The next logical step in the evolution of TAVI is the expansion of treatment indications to low risk patients, and 3 randomized controlled trials (PARTNER 3 [NCT02675114], Medtronic Evolut transcatheter aortic valve replacement in low risk patients [NCT02701283], and NOTION-2 [NCT02825134]) comparing TAVI to SAVR in this cohort are already underway with initial outcomes imminent (Table 1). Although the volume of TAVI procedures is already growing exponentially,4,5 demographic analyses suggest that the addition of low-risk patients as legitimate TAVI candidates will increase the potential number of procedures by at least 50%.6 Successful treatment of this cohort will require TAVI teams to set new standards in terms of clinical outcomes and cost effectiveness. In this review, we present the main challenges that need to be addressed before TAVI can be applied as a standard treatment for low risk patients:
- •
Reducing periprocedural morbidity and mortality.
- •
Management of concomitant coronary artery disease.
- •
Concerns regarding long-term valve durability.
- •
Cost effectiveness.
Randomised Clinical Trials of TAVI vs SAVR in Low Surgical Risk Patients
| Trial | Inclusion criteria | Primary outcomes | Primary completion date |
|---|---|---|---|
| Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients (NCT02701283) | Severe symptomatic ASSevere asymptomatic AS with velocity ≥ 5.0 m/sec/mean gradient ≥ 60 mmHg/abnormal exercise tolerance test/LVEF <50%Documented Heart Team agreement of low risk for SAVR (defined as <3% predicted 30 day mortality) | Two year all-cause mortality or disabling stroke | April 2021 |
| Comparison of Transcatheter Versus Surgical Aortic Valve Replacement in Younger Low Surgical Risk Patients With Severe Aortic Stenosis (NOTION-2) (NCT02825134) | Age 75 years or youngerSevere calcific AS (confirmed by dobutamine stress echo if LVEF <50% or low flow AS)Symptoms related to ASSTS score <4%Suitable for both SAVR and transfemoral TAVILife expectancy> 1 year after the intervention | One year composite rate of all-cause mortality, myocardial infarction and stroke | June 2020 |
| PARTNER 3 trial –The Safety and Effectiveness of the SAPIEN 3 Transcatheter Heart Valve in Low Risk Patients With Aortic Stenosis (NCT02675114) | Severe calcified aortic stenosisHeart Team agreement that patient is low risk for operative mortality with STS score <4 | One year all-cause mortality, all stroke, and rehospitalisation | January 2020 |
AS, aortic stenosis; LVEF, left ventricular, ejection fraction; SAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation.
Recent US data demonstrate that the availability of TAVI has had a significant effect on the risk profile and outcomes of SAVR, in high-volume TAVI centres—risk scores of SAVR patients have decreased with a concomitant reduction in periprocedural mortality.7 Furthermore, 30-day mortality was 0% in a recent multicenter registry of 200 low-risk patients undergoing TAVI,8 indicating that the procedure is a legitimate alternative to SAVR in this cohort. However, TAVI is associated with an increased risk of important periprocedural complications—paravalvular leak (PVL), permanent pacemaker (PPM) implantation and peripheral vascular events2,3,9—and further refinements will be necessary for TAVI to emerge as the gold standard treatment for AS in low-risk patients.
Paravalvular LeakModerate or severe PVL was reported in over 20% of patients receiving early generation TAVI devices10 and was associated with reduced long-term survival.11 Although some data suggest that even mild PVL is associated with adverse prognosis,12 the clinical impact of PVL has decreased significantly due to 2 major changes:
- •
Routine use of multislice computed tomography (CT) for annular sizing.
- •
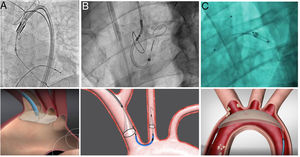
Improved design of newer generation valves incorporating skirts, modified frames, and full retrievability (Figure 2).
Figure 2.Currently available transcatheter heart valves. Top left, Evolut PRO (Medtronic, Minnesota, United States). Top right, ACURATE Neo (Boston Scientific, Massachusetts, United States). Bottom left, SAPIEN 3 Ultra (Edwards Lifesciences, California, United States). Bottom right, LOTUS Edge (Boston Scientific).
(0.12MB).
Rates of significant PVL are now as low as 1.5% for latest generation valves13 with important benefits for low-risk patients who have a higher prevalence of bicuspid aortic valves and longer life expectancy following TAVI.
Permanent Pacemaker ImplantationPPM implantation is the most frequent complication of TAVI, affecting 13% of patients in a pooled analysis of 49 studies (n=16063) using first generation devices.14 Unlike other complications, this has not improved significantly with the latest generation of CoreValve self-expanding valves15 and latest iterations of the Sapien balloon expandable valve may be associated with higher PPM implantation rates.16,17 The prognostic impact of PPM implantation following TAVI remains controversial: a meta-analysis of 11 studies (n=7032) showed no impact on 1-year mortality18 but a later Transcatheter Valve Therapy registry report (n=9875) suggested a 31% increase in 1-year mortality when a PPM was required after TAVI.19 Whether these data (derived from intermediate and high-risk octogenarians) are relevant to low-risk patients is unclear. While low risk patients have a lower baseline prevalence of conduction system disorders, detrimental effects of prolonged ventricular pacing20 or new onset conduction disturbances21 are likely to be more evident in this cohort.
There has been much progress in recent years to identify predictors of PPM requirement following TAVI:
- •
Preprocedural: first-degree atrioventricular (AV) block, left anterior hemiblock, right bundle branch block, male sex.
- •
Procedural: self-expanding devices, intraprocedural heart block, depth of implantation22.
High implantation of balloon expandable valves is likely to reduce PPM implantation rates in patients at particular risk23,24 and a significant proportion of PPM implants following TAVI may be avoidable. Rates of pacemaker dependency after TAVI range from 27% to 68%25,26 and many patients receiving a PPM recover normal AV conduction over 30 day follow-up.24 The likelihood of PPM dependency is very high for advanced AV block but negligible (< 1%) for new onset left bundle branch block or first-degree AV block,27 suggesting that significant reduction in the need for PPM implantation could be achieved with improved adherence to guidelines for the management of periprocedural conduction disturbances and more liberal use of a “watchful waiting” approach. This issue is particularly relevant for low-risk patients, since the main factor driving “unnecessary” prophylactic PPM implantation is the attempt to shorten the duration of hospital admission– a specific consideration that will only increase in low-risk groups (vide infra).
Periprocedural StrokePeriprocedural stroke increases TAVI mortality 5-fold and is associated with significant disability and reduced quality of life in survivors28—the incidence in the 30 days following TAVI ranges from 2% to 5% and was 2.8% in a meta-analysis of 58 studies.29 The PARTNER II trial comparing TAVI to SAVR in intermediate-risk patients showed no difference in stroke rates at the 30-day, 1- or 2-year follow-up2 while stroke rates were lower after TAVI at the 30-day, 1- and 2-year follow-up in the SURTAVI trial (although this difference was only statistically significant at 30 days).3 Importantly, quality of life measures suggested swifter improvement after stroke related to TAVI than SAVR,30 while a retrospective study comparing patients with previous stroke (excluded from most randomized trials) undergoing TAVI (n=839) and SAVR (n=2695) found no difference in periprocedural stroke rates (1.4% vs 1.2%, P=.642).31
A related issue is the significance of “silent” stroke, ie, new ischemic brain lesions detected by systematic cerebral imaging.32,33 Studies have shown a variable (though consistently high) frequency of new ischemic brain lesions following TAVI, with a frequency of 77.5% in a meta-analysis of 25 studies (n=1225) and multiple (mean=4.2) lesions in the majority (59.5%) of patients.34 Unlike the general population,35 the significance of “silent” stroke following TAVI is uncertain, since most patients with new ischemic lesions have no neurological or cognitive impairment.36
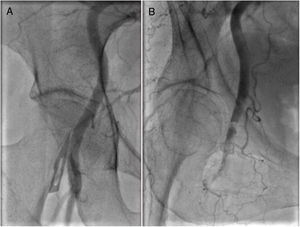
Periprocedural strokes are believed to be embolic in nature, arising from calcified and atherosclerotic material in the valve leaflets and aortic arch, and efforts to reduce TAVI-related stroke focus principally on the use of cerebral embolic protection devices. Several devices are currently in use: the Embrella Embolic Deflector Device and Embol-X system (Edwards Lifesciences, Irvine, California, United States), the Triguard system (Keystone Heart, Herzliya, Israel), and the Sentinel embolic protection device (Claret Medical, Santa Rosa, California, United States) (Figure 3). While all of these devices are safe, limited effectiveness data are available. Thus, while individual studies and meta-analyses have consistently shown reduction in total cerebral lesion volume associated with the use of embolic protection devices, the impact on clinical event rates remains unclear.37 While 1 meta-analysis (8 studies, n=1285) demonstrated a reduction in stroke rate at the 30-day follow-up (odds ratio, 0.55; 95% confidence interval, 0.31-0.98; P=.04),38 this outcome was driven mainly by a single nonrandomized study.39
An interesting concept under preliminary evaluation is the use of combined cerebral protection devices to cover both the carotid (anterior) and vertebral (posterior) circulation. A recent report combining the use of the Sentinel device (protecting the right carotid, left carotid and right vertebral arteries) with a single left vertebral filter (Wirion, Allum Medical Inc, Caesarea, Israel) demonstrated equal distribution of debris in both devices.40 This approach may be more clinically beneficial given the link between new lesion volume and cognitive decline.
Other measures that may reduce periprocedural stroke rates include lower profile devices, and delivery systems, and improved steerability to minimize unnecessary aortic contact during delivery and deployment. Similarly, preliminary balloon valvuloplasty is no longer routine and reserved for a small proportion (< 10%) of cases.41
As the focus turns to younger, lower-risk patients, the usefulness of cerebral protection devices may be more specifically defined for patients with specific embolic risk markers, including left atrial thrombus, premature cerebrovascular disease, bulky valve leaflets, and aortic arch atheroma.
Access-related Vascular ComplicationsThe rate of major vascular complications is consistently above 10% in extreme and high-risk populations (Figure 4). However, increasing operator experience and improvements in valve design (permitting progressive reduction in sheath sizes to 14/16 Fr) have significantly reduced the risk in intermediate risk patients (PARTNER II=7.9%,2 SURTAVI=6.0%3, Sapien 3 registry=6.1%16).
Predictors of vascular complications include the sheath-to-artery ratio, presence of circumferential calcification, severe tortuosity, failure of preclosure using percutaneous devices, and the preceding familiarity of TAVI operators with femoral puncture techniques. While the current generation of TAVI operators came from a background of transfemoral coronary interventions, the training of future TAVI operators will be in those with a background of mainly radial procedures. Increasing use of radial access is associated with a higher rate of complications when femoral procedures are performed,42 which may result in a flatter learning curve for new TAVI operators when performing large bore femoral access procedures.
Other measures that may help reduce access-related complications are ultrasound-guided femoral access,43 improved sheath and delivery system design, use of CT reconstruction to identify calcification, tortuosity and accurate ilio-femoral size, and the use of transaortic,44 transaxillary,45 and transcaval46 access for patients with challenging iliofemoral anatomy.
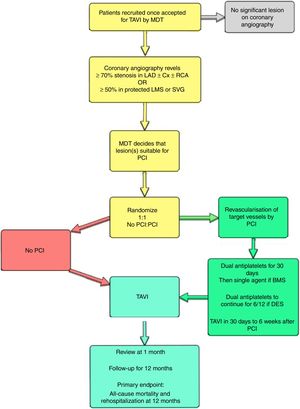
MANAGEMENT OF CONCOMITANT CORONARY ARTERY DISEASEAtherosclerosis and degenerative AS share many risk factors, and it is unsurprising that coronary artery disease (CAD) is one of the most common comorbidities in the TAVI population, with a prevalence of ≈ 50% in large registries47 and 75% in PARTNER 1.48,49 However, unlike other comorbidities—frailty,50 chronic kidney disease,51 obstructive lung disease and atrial fibrillation52—that influence prognosis after TAVI, CAD is potentially treatable. While coronary revascularization prior to aortic valve surgery improves outcomes,53 it remains unclear whether the same is true for all TAVI recipients.54 Possible explanations include the shorter life expectancy of current TAVI patients, the competing risk of death from other comorbidities, and the heterogenous nature of CAD. Indeed, several retrospective studies55,56 have confirmed that patients with moderate or severe CAD (SYNTAX score> 22) have worse prognosis after TAVI and failure to achieve a residual SYNTAX score <8 prior to TAVI is associated with an increase in overall mortality (odds ratio, 1.69, 95% confidence interval, 1.26-2.28; P <.001).57 Currently there are no accepted recommendations concerning the necessity or extent of revascularization in TAVI patients with concomitant CAD, and outcomes of the ACTIVATION trial (ISRCTN75836930)58 addressing this question are keenly awaited (Figure 5).
ACTIVATION trial flow chart (ISRCTN 75836930), the first randomized trial of coronary revascularization in transcatheter aortic valve implantation candidates with concomitant coronary disease. BMS, bare metal stent; Cx, circumflex artery; DES, drug-eluting stent; LAD, left anterior descending artery; LMS, left main stem; MDT, multidisciplinary team; PCI, percutaneous coronary interventions; RCA, right coronary artery; TAVI, transcatheter aortic valve implantation; SVG, saphenous vein graft.
Questions concerning the optimal management of CAD are of greater importance in low-risk TAVI patients since untreated CAD is likely to be of more prognostic significance. Furthermore, coronary access and PCI may be more technically challenging after TAVI,59 supporting the argument for pre-emptive revascularization prior to TAVI.
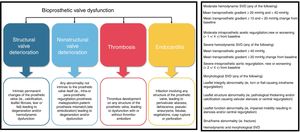
REASSURING CONCERNS REGARDING VALVE DURABILITYThe questions of long-term durability and risk of structural valve dysfunction (SVD) are of utmost importance when considering the prospect of TAVI in low-risk patients with longer life expectancy than those studied so far. SVD may arise as a result of degeneration, calcification, thrombosis, infection and pannus formation and lead to valve-related death or the need for reintervention. International definitions of SVD for clinical and research purposes have only recently been published60 (Figure 6).
Pathophysiological mechanisms of bioprosthetic valve dysfunction (left) and European consensus definitions of structural valve dysfunction (right). SVD, structural valve dysfunction. Adapted with permission from Capodanno et al.60.
A systematic review of observational studies on the durability of surgical bioprosthetic valves demonstrated SVD-free survival of 94%, 82%, and 52% at 10, 15, and 20 years, respectively.61 Although limited, the available data concerning mid-term durability of TAVI devices are similarly reassuring. In PARTNER 1A, no patient who survived to 5 years required SAVR due to SVD in both the TAVI or SAVR arms and echocardiographic assessment confirmed stable and equivalent valve performance in surviving patients.11 Recently, long-term follow-up data from the FRANCE 2 registry60 reported cumulative incidence of 2.5% and 13.3% severe and moderate/severe SVD at 5 years while in the OBSERVANT registry,62 the corresponding 8-year figures were 2.4% and 8.3%. Although these data are reassuring, longer term follow-up to 10 years and beyond in large scale registries (including second and third generation valves) will be required before routine application of TAVI in low-risk younger patients. A related issue concerns recent reports regarding computed tomography detection of hypoattenuation and leaflet thickening, present in up to 15% of patients undergoing CT.63,64 These findings, which resolve with anticoagulation with either vitamin K antagonists or unfractionated heparin are accepted to represent leaflet thrombosis. Such thrombosis may lead to early valve failure presenting as increased transvalvular gradients and dyspnea, thromboembolism and stroke, but can also be a chance finding without sequelae.65,66 However, current clinical practice does not mandate routine post-TAVI CT imaging as there is no proven correlation with outcome. This could have significant implications and could limit the potential expansion of transcatheter therapies in the lower-risk population.
COST EFFECTIVENESSThe debate over the cost effectiveness of TAVI is longstanding and far from settled. While TAVI devices are much more expensive than surgical valves, overall treatment costs are lower as a result of shorter hospital stay and a lesser need for intensive care and rehabilitation services. Economic analyses based on 12-month outcomes show that transfemoral TAVI for high risk patients matches US health care cost-effectiveness standards.67,68 Similar analyses demonstrate reduction of overall costs at 2 years after TAVI compared with SAVR in intermediate-risk patients while TAVI is associated with better quality adjusted outcomes and may emerge as the economically dominant treatment strategy in this group [Cohen DJ, presented at TCT 2017]. The converse may be true in low-risk patients since outcomes will be even better for both TAVI and SAVR while the excess procedural costs of TAVI will remain the same and differences in length of hospital stay and rehabilitation requirement diminish. Importantly, these projections are based on US costings and may not apply in other health care settings.
Key requirements to further improve the cost effectiveness of TAVI include cheaper devices (which may arise in response to competitive market forces), lower complication rates, reduced length of stay (same-day or next-day discharge may be feasible),69,70 and further procedural streamlining (reduced staff requirements and nurse-led sedation in selected patients).71
CONCLUSIONSContinued technical and procedural refinement coupled with the very understandable preference of patients to undergo a less invasive procedure mean that extension of TAVI to low-risk patients is inevitable. However, as we have described, many questions remain before this becomes acceptable standard practice. Only when key issues concerning durability and the elimination of major complications have been addressed can TAVI challenge SAVR as the gold standard treatment for all patients with severe AS. Even then, demonstrable cost effectiveness will be required before adoption by public health care systems. Until these issues are resolved, Heart Team discussions concerning the optimal management of low risk patients with severe AS must consider the remaining limitations of TAVI and make judicious, evidence-based decisions for individual patients.
CONFLICTS OF INTERESTNone declared.