Atrial fibrillation (AF) is linked to heart failure (HF). However, little has been published on the factors that may precipitate the onset of HF in AF patients. We aimed to determine the incidence, predictors, and prognosis of incident HF in older patients with AF with no prior history of HF.
MethodsPatients with AF older than 80 years and without prior HF were identified between 2014 and 2018.
ResultsA total of 5794 patients (mean age, 85.2±3.8 years; 63.2% women) were followed up for 3.7 years. Incident HF, predominantly with preserved left ventricular ejection fraction, developed in 33.3% (incidence rate, 11.5-100 people-year). Multivariate analysis identified 11 clinical risk factors for incident HF, irrespective of HF subtype: significant valvular heart disease (HR, 1.99; 95%CI, 1.73-2.28), reduced baseline left ventricular ejection fraction (HR, 1.92; 95%CI, 1.68-2.19), chronic pulmonary obstructive disease (HR, 1.59; 95%CI, 1.40-1.82), enlarged left atrium (HR 1.47, 95%CI 1.33-1.62), renal dysfunction (HR 1.36, 95%CI 1.24-1.49), malnutrition (HR, 1.33; 95%CI, 1.21-1.46), anemia (HR, 1.30; 95%CI, 1.17-1.44), permanent AF (HR, 1.15; 95%CI, 1.03-1.28), diabetes mellitus (HR, 1.13; 95%CI, 1.01-1.27), age per year (HR, 1.04; 95%CI, 1.02-1.05), and high body mass index for each kg/m2 (HR, 1.03; 95%CI, 1.02-1.04). The presence of incident HF nearly doubled the mortality risk (HR, 1.67; 95%CI, 1.53-1.81).
ConclusionsThe presence of HF in this cohort was relatively frequent and nearly doubled the mortality risk. Eleven risk factors for HF were identified, expanding the scope for primary prevention among elderly patients with AF.
Keywords
Atrial fibrillation (AF) and heart failure (HF) have a high prevalence and increasing incidence.1–4 Both cardiovascular diseases affect approximately 2% of the population but are age-dependent, affecting up to 1 in 10 patients older than 80 years.5–8 Prior studies have highlighted that AF can precipitate HF and that HF can be a risk factor for AF. Moreover, both entities frequently coexist.9 In the Framingham cohort,10 37% of the patients with AF had HF and 57% of those with HF had AF. Moreover, the prevalence of AF prevalence (from 5% to 50%) rises as New York Heart Association (NYHA) class worsens.11
There is a complex bidirectional relationship between both AF and HF and several risk factors are common to both diseases.5,8,12,13 Indeed, until recently, the underlying pathophysiological mechanisms of the 2 diseases were considered common.7,14 However, a recent study based on the Monica Risk, Genetics, Archiving, and Monograph/Biomarker for Cardiovascular Risk Assessment in Europe consortium, with 58 693 individuals from 5 cohort studies (DanMONICA, FINRISK, Moli-sani, Northern Sweden, and Scottish Heart Health Extended Cohort),14 showed that, although AF and HF share pathophysiological similarities and frequently coexist, there are distinct risk profiles that may be related to specific pathophysiological aspects of the 2 diseases.15–18 Consequently, the prevention of AF and HF in individuals who already have 1 of these diseases needs to be addressed rigorously. This opens up a new opportunity for prevention but remains challenging. In this regard, traditional modifiable risk factors provide opportunities for interventions.
In patients with AF, little focus is placed on risk stratification and prevention of HF, especially in the subgroup of older patients, who are at high risk. Modifiable risk factors for HF should be rigorously targeted in this population to reduce the risk of incident HF, which is associated with an increased risk of mortality. However, there have been scarce reports of the factors that can precipitate the onset of HF in AF patients. In this study, our objective was to evaluate the incidence, significant clinical predictors and prognosis of incident HF in older patients with AF.
METHODSPatient populationWe performed a retrospective observational cohort study including all consecutive patients aged ≥ 80 years (n=6489) with a diagnosis of AF between January 2014 and January 2018 in the health area of Vigo (Galicia, Spain). The CardioCHUVI- AF registry was used (ClinicalTrials.gov identifier: NCT04364516). Diagnoses consisted of the first diagnosis of AF, which was considered the inclusion date. All diagnoses and procedures during follow-up were verified by 2 cardiologists. AF diagnosis was confirmed by the presence of a compatible electrocardiogram. Clinical, laboratory, and therapeutic data were collected in an encoded database. We excluded patients with a prior history of HF (n=624), together with those with missing baseline and follow-up data (n=71); therefore, the final cohort was composed of 5794 AF patients. The study was conducted following the principles of the Declaration of Helsinki and was approved by the local ethics committee (Autonomous Committee of Research Ethics of Galicia, code HAC-ACO-2018-01, registry 2018/258).
Follow-up and study outcomesHF status and death during follow-up were ascertained retrospectively from inclusion to October 2020. The primary outcome of interest was HF incidence at follow-up. Incident HF was defined as the occurrence of a new diagnosis of clinical HF observed on follow-up visits. HF diagnosis was based on the presence of both signs and symptoms due to a documented functional or structural cardiac abnormality, according to current European Society of Cardiology guidelines.19 Among patients with incident HF, we used left ventricular ejection fraction (LVEF) at the time of incident HF diagnosis to identify HF subtypes: HF with preserved LVEF (heart failure with preserved ejection fraction [HFpEF]; LVEF>50%), HF with mildly reduced LVEF (heart failure with mid-range ejection fraction [HFmrEF]; LVEF 40%-49%), and HF with reduced LVEF (heart failure with reduced ejection fraction [HFrEF]; LVEF ≤ 40%). HF diagnosis could be made in the outpatient clinic or during hospitalization. Both cardiologists confirmed the diagnosis, with particular care in HFpEF, in which they confirmed the presence of left ventricular diastolic dysfunction/raised left ventricular filling pressures on the echocardiogram, as well as raised natriuretic peptides.
AF management was carefully recorded, with special emphasis on treatment to restore sinus rhythm. Overweight was defined as body mass index (BMI) ≥ 25, moderate or severe valvular heart disease was considered significant, and renal impairment was defined as estimated renal clearance by Renal clearance by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) of<60mL/min/1.73m2. Anemia was defined as hemoglobin concentration less than 12g/dL in women or 13g/dL in men, while a diagnosis of malnutrition was made using Controlling Nutritional Status (CONUT) score.20 Atrial size was measured based on echocardiography American guidelines.21 Secondary outcomes that were compared between patients with vs without incident HF consisted of all-cause death, cardiovascular mortality, stroke/thromboembolism, and bleeding events. For these endpoints, we used the definitions for clinical trials developed by the Standardized Data Collection for CV Trials Initiative (SCTI) and the US Food and Drug Administration (FDA),22 together with the statements of the International Society on Thrombosis and Haemostasis (ISTH).23
Statistical analysisThe study population was stratified by the incidence of HF on follow-up: patients with vs without HF. The baseline characteristics of study participants are described as mean±standard deviation for continuous variables, and as counts (proportions) for categorical variables. Differences in baseline characteristics between the 2 groups were compared using unpaired t-tests and chi-square tests for continuous and categorical variables, respectively.
The incidence of HF was estimated using weighted cumulative incidence curves after using a Fine-Gray proportional subdistribution hazards model, with death serving as a competing risk. The proportionality assumption was verified by testing for an interaction between the exposure variable and time, and no relevant violations were found. Effect estimates were reported as subdistribution HRs (sHRs) along with 95% confidence intervals (95%CIs). Multivariate adjustment was developed and included all variables showing a significant clinical or statistical association with HF on univariate analysis (table 2) in addition to age and sex.
We evaluated the association between incident HF and mortality using unadjusted Cox proportional hazards models with the HF event modeled as a time-dependent covariate. The proportional hazards assumption was checked using plots of the log of the negative log of the survival function against the log of time. Multivariate Cox logistic regression (backward stepwise method) was performed after adjustment for age, sex, CHA2DS2-VASc score, HAS-BLED score, and anticoagulation therapy.
Statistical analyses were conducted using STATA software, version 15 (Stata Corp, College Station, United States). A 2-sided P<.05 was considered statistically significant.
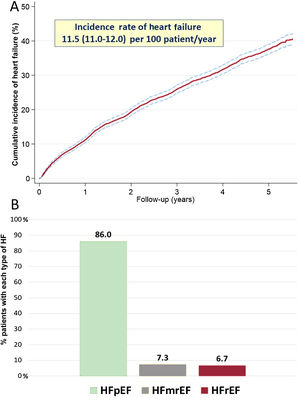
RESULTSBaseline characteristics and incident HFOf 5794 consecutive AF patients (mean age, 85.2±3.8 years; 63.2% women), 1930 patients (33.3%) developed incident HF during a median follow-up of 3.7 [1.9-5.1] years, with an incidence rate of 11.5 (95%CI, 11.0-12.0) per 100 patient-years (figure 1A). Of these, 86.0% (n=1661) developed HFpEF, 7.3% (n=140) HFmrEF, and 6.7% (n=129) HFrEF (figure 1B). The baseline characteristics of the study participants are shown in table 1.
Baseline characteristics
| Study population | |
|---|---|
| Age, y | 85.2±3.8 |
| Female sex | 1386 (63.2) |
| Current smoking | 31 (0.5) |
| Body mass index, kg/m2 | 29.2±3.9 |
| Hypertension | 3669 (63.3) |
| Diabetes mellitus | 1084 (18.7) |
| Dyslipidemia | 2251 (38.9) |
| Peripheral artery disease | 219 (3.8) |
| Ischemic heart disease | 636 (11.0) |
| Prior stroke | 461 (8.0) |
| Prior cancer | 455 (7.9) |
| COPD | 623 (10.8) |
| Anemia | 1453 (25.1) |
| eGFR<60 mL/min/1.73 m2 | 2515 (43.4) |
| Malnutrition | 2,125 (36.5) |
| AF type | |
| Paroxysmal | 1033 (17.8) |
| Persistent | 403 (7.0) |
| Permanent | 4358 (75.2) |
| Baseline LVEF ≥ 50% | 476 (8.2) |
| LA enlargement | 2514 (43.4) |
| Significant heart valve disease | |
| Aortic stenosis | 252 (4.3) |
| Aortic regurgitation | 25 (0.4) |
| Mitral stenosis | 16 (0.3) |
| Mitral regurgitation | 185 (3.2) |
| CHA2DS2-VASc, points | 3.8±1.1 |
| HAS-BLED, points | 2.8±1.0 |
| Anticoagulation therapy | 4620 (79.7) |
| Type of anticoagulant drug | |
| DOAC | 730 (12.6) |
| VKA | 3825 (66.0) |
| Heparin | 65 (1.1) |
| Antiarrhythmic drugs | 245 (4.2) |
| Beta-blocker | 1803 (31.1) |
| CCB | 91 (1.6) |
| Digoxin | 854 (14.7) |
| RAS inhibitor | 2674 (46.2) |
| Statin | 1985 (34.3) |
AF, atrial fibrillation; CCB, calcium channel blocker; COPD, chronic pulmonary obstructive disease; DOAC, direct oral anticoagulants; eGFR, estimated glomerular filtrate rate by Chronic Kidney Disease Epidemiology Collaboration; LA, left atrium; LVEF, left ventricular ejection fraction; RAS, renin-angiotensin-system; VKA, vitamin K antagonist.
The baseline risk factors significantly associated with the subsequent development of HF in the univariate analysis are shown in table 2. In the multivariate analysis, independent predictors of subsequent HF were age, BMI, malnutrition, diabetes mellitus, baseline LVEF, valvular heart disease, left atrial enlargement, permanent AF, chronic pulmonary obstructive disease (COPD), anemia, and estimated glomerular filtrate rate (all P<.05). Renin-angiotensin-aldosterone system inhibitors were a protective factor for incident HF in this cohort (P=.048). Through the different values of these variables, the risk of developing incident HF could be stratified (figure 1 of the supplementary data).
Risk factors associated with incident heart failure
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| sHR | 95%CI | P | sHR | 95%CI | P | |
| Age, per year | 0.99 | 0.98-1.00 | .133 | 1.04 | 1.02-1.05 | <.001* |
| Female sex | 0.94 | 0.86-1.03 | .198 | 1.01 | 0.92-1.12 | .764 |
| Body mass index, per kg/m2 | 1.03 | 1.01-1.04 | <.001 | 1.03 | 1.02-1.04 | <.001* |
| Smoking | 1.31 | 0.81-2.11 | .272 | 1.68 | 0.97-2.92 | .064 |
| Malnutrition | 1.39 | 1.27-1.52 | <.001 | 1.33 | 1.21-1.46 | <.001* |
| Hypertension | 1.04 | 0.95-1.14 | .414 | 0.96 | 0.87-1.06 | .401 |
| Dyslipidemia | 1.06 | 0.97-1.16 | .193 | 0.95 | 0.86-1.04 | .236 |
| Diabetes mellitus | 1.17 | 1.04-1.31 | .007 | 1.13 | 1.01-1.27 | .042* |
| Peripheral artery disease | 1.11 | 0.88-1.41 | .370 | 1.03 | 0.81-1.31 | .798 |
| Coronary artery disease | 1.18 | 1.02-1.35 | .022 | 1.03 | 0.89-1.19 | .670 |
| Valvular heart disease | 2.52 | 2.21-2.87 | <.001 | 1.99 | 1.73-2.28 | <.001* |
| Prior cancer | 1.00 | 0.84-1.19 | .999 | 0.97 | 0.81-1.16 | .758 |
| COPD | 1.60 | 1.41-1.82 | <.001 | 1.59 | 1.40-1.82 | <.001* |
| Liver disease | 0.96 | 0.67-1.38 | .829 | 0.92 | 0.63-1.36 | .688 |
| Anemia | 1.40 | 1.26-1.54 | <.001 | 1.30 | 1.17-1.44 | <.001* |
| eGFR<60 mL/min/1.73 m2 | 1.44 | 1.32-1.57 | <.001 | 1.36 | 1.24-1.49 | <.001* |
| Permanent AF | 1.15 | 1.04-1.28 | .009 | 1.15 | 1.03-1.28 | .013 |
| Baseline LVEF<50% | 2.59 | 2.28-2.95 | <.001 | 1.92 | 1.68-2.19 | <.001* |
| Left atrial enlargement | 1.88 | 1.72-2.06 | <.001 | 1.47 | 1.33-1.62 | <.001* |
| Anticoagulation | 1.19 | 0.98-1.44 | .077 | 1.14 | 0.93-1.39 | .212 |
| Beta-blockers | 1.07 | 0.97-1.18 | .177 | 1.06 | 0.96-1.17 | .235 |
| RAAS inhibitors | 0.94 | 0.86-1.03 | .163 | 0.91 | 0.83-0.99 | .048* |
95%CI, 95% confident interval; eGFR by CDK-EPI, estimated glomerular filtrate rate by Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; LA, left atrium; LVEF, left ventricular ejection fraction; RAAS, renin-angiotensin-aldosterone system inhibitors; sHR, subdistribution hazard ratio.
* Statistically significant values.
For outcomes of different HF subtypes, the baseline risk factors that independently predicted the incidence of the different types of HF (HFpEF, HFmrEF, and HFrEF) did not differ from those identified for overall HF.
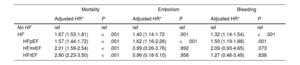
Events in patients with AF and incident HFThe incidence of major adverse clinical events, such as all-cause mortality, embolism and bleeding, was significantly higher in patients with AF with subsequent incident HF than in those without HF during follow-up. However, the association between incident HF and risk of bleeding and embolic events was attenuated and was not significant after adjustment for potential confounders in patients with HFmrEF and HFrEF (table 3). Incident HFpEF remained an independent risk factor for both embolism and bleeding (figure 2)
Major adverse clinical events stratified by heart failure groups
| Mortality | Embolism | Bleeding | ||||
|---|---|---|---|---|---|---|
| Adjusted HR* | P | Adjusted HR* | P | Adjusted HR* | P | |
| No HF | ref | ref | ref | ref | ref | ref |
| HF | 1.67 (1.53-1.81) | <.001 | 1.40 (1.14-1.72 | .001 | 1.32 (1.14-1.54) | <.001 |
| HFpEF | 1.57 (1.44-1.72) | <.001 | 1.62 (1.16-2.26) | <.001 | 1.50 (1.19-1.88) | .001 |
| HFmrEF | 2.01 (1.59-2.54) | <.001 | 0.99 (0.26-3.76) | .992 | 2.09 (0.93-4.65) | .073 |
| HFrEF | 2.80 (2.23-3.50) | <.001 | 0.96 (0.18-5.10) | .958 | 1.27 (0.46-3.49) | .638 |
95%CI, 95% confidence interval; No HF, patients not developing de novo heart failure; HFmrEF, heart failure with mid-range ejection fraction); HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction, sHR, hazard ratio.
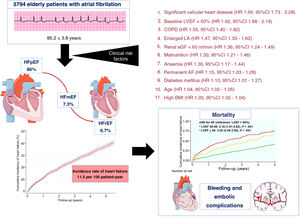
Central illustration. Follow-up of older patients with atrial fibrillation. Risk factors and clinical outcomes. 95%CI, confidence interval; AF, atrial fibrillation; BMI, body mass index; eGFR, estimated glomerular filtrate rate by Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; LA, left atrium; LVEF, left ventricular ejection fraction, HFmrEF, heart failure with mid-range ejection fraction); HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio.
The present study is the first to analyze the incidence, predictors, and prognosis of incident HF in the follow-up of a large cohort of elderly patients with AF (figure 2).
In this observational study, covering a complete health care setting for a community of older patients with AF patients, our main findings were as follows: a) the incidence rate of de novo HF in patients with AF was 11.5-100 people-year; b) de novo HF developed in 33.3% of patients; c) most patients were diagnosed in the first 5 years of follow-up and mainly had HFpEF; d) the following 11 risk factors for HF were identified, ranked in order from most to least relevant: significant valvular heart disease, reduced baseline LVEF, COPD, enlarged LA, renal dysfunction, malnutrition, anemia, permanent AF, diabetes mellitus, older age, and higher BMI; e) the likelihood of developing HF can be calculated by stratifying these risk factors, while renin-angiotensin-aldosterone system inhibitors proved to be a protective factor; f) the presence of HF in elderly AF patients with no prior history of HF increased mortality by 1.67-fold; g) bleeding increased by 32% and embolism by 40% in patients with incident HF.
Comparison with existing literatureThe present study examined the largest cohort to date of older patients (n=5794) with AF without prior HF. Pandey,24 Miyasaka3 and Wong et al.25 prospectively analyzed 6545, 3288, and 3167 patients with AF, respectively, but irrespective of age (the mean age was 74, 71, and 61.5 years, respectively).
The rate of incident HF development in our study (11.5%; 115.26 per 1000 people-year) was higher than figures reported in the literature (ranging from 1%26,27 to 4.4%3,24,28,29), although this discrepancy could be because the our cohort was very old and AF is much more common in persons of advanced age than in the younger population. Schnabel et al.28 stratified their cohort study by age of AF onset and reported that the incidence rate was twice as high in the population older than 70 years (41% per 10 years vs 20% in younger).
Secondly, more older elderly patients than expected were diagnosed in the first 5 years after receiving a diagnosis of AF, in contrast with the situation in the general population, in which most HF diagnoses were made between 4 and 10 years of follow-up.28,30
Many other studies have attempted to ascertain the risk factors for incident HF and its progression: Chatterjee et al.29 described 4 modifiable risk factors in women with new-onset AF, which, except for diabetes, were the same as those in in a Sweden cohort25: hypertension (systolic blood pressure>120mmHg), tobacco (active smoking), and obesity. In our study, neither hypertension nor ischemic heart disease, traditionally considered risk factors, were identified as risk factors. Insufficient data on blood pressure control or extent of ischemia may have an impact. Eggimann et al.27 detected 8 predictors of HF hospitalizations among 1193 patients with AF, including laboratory results such as brain natriuretic peptide, diabetes mellitus, and again, overweight. Miyasaka et a.3 reported that their multivariable analyses revealed that 15 variables were related to HF development in people with AF, including all cardiovascular risk factors, COPD, and paroxysmal AF. Finally, Pandey et al.24 identified 6 risk factors of incident HF, 4 of which were also identified in our work (age, renal dysfunction, valvular disease, and permanent AF); however, these authors only differentiated between reduced and preserved ejection fraction, with a large percentage of missing LVEF values.
Of note, none of these risk factors differed according to age, even though the 2 entities (AF and HF) are fairly common in older people11,31 and have been always linked.32 In our study, we identified 10 modifiable risk factors, of which 7 of which were previously described in the general population3,25,27,29 (age, BMI, low LVEF, LA enlargement, renal dysfunction, COPD, and indirectly, significant valvular heart disease). All of these factors were the same for each HF subgroup (preserved, mildly reduced, and reduced LVEF). However, except for overweight, no other cardiovascular risk factors or vasculopathy were related to the risk of developing HF in the older population.
Clinical implicationsAF has been related to HF decompensation and progression,33 as well as to cognitive impairment and stroke.34 For these reasons, the disease is associated with a high proportion of admissions,24 morbidity,35 mortality5,26 and therefore health costs.9,36
Due to the above, and its close relationship with HF,37 HF in the AF population has been recognized as the “most common nonfatal event” increasing cardiovascular mortality.3,26,29 In our study, incident HF nearly doubled the risk of death (HR, 1.67; 95%CI, 1.53-1.81), with the risk being higher as ejection fraction decreased. Moreover, relevant clinical events, such as embolism and bleeding, were markedly higher among patients with incident HF (40% and 32% more, respectively). In this elderly cohort with AF, renin-angiotensin-aldosterone system inhibitors were a protective factor for incident HF, suggesting the advisability of their use from the onset of this entity.
In order of relevance, the conditions directly related to a higher probability of incident HF in our older cohort with AF were the following: significant valvular heart disease, reduced LVEF at baseline, the presence of COPD, enlarged left atrium, chronic kidney disease, malnutrition, anemia, permanent AF, diabetes mellitus, and higher age or BMI. Recognition of these clinical risk factors, which were independent of HF subtype, would allow us to identify an older population at risk of developing HF, and thus to apply therapeutic algorithms for primary prevention.
LimitationsThe present study analyzed a cohort of 5794 patients older than 80 years with AF and without prior HF. Moreover, the patients were followed up for 3.7 years. Endpoints were adjudicated based on the criterion of a physician. Eleven risk factors, most which are modifiable, were identified, allowing stratification of the cumulative incidence of HF over time among patients with AF. Furthermore, incident HF was related to the risk of mortality, embolism, and bleeding.
This study has some limitations. The results emanate from a retrospective study including patients older than 80 years with a diagnosis of AF in a single tertiary hospital, with the limitations inherent to this type of design. Moreover, and according to the inclusion criteria, the patients usually had AF before the start of follow-up.
Although we report data on anticoagulation, beta-blockers and renin-angiotensin system inhibitors, we have no data on other treatments of interest, such as sodium-glucose cotransporter-2 inhibitors or aldosterone antagonists. In addition, although we used a multivariable model to adjust for potential confounders, some residual confounding factors might persist. In this regard, we have no data on N-terminal pro-brain natriuretic peptide or specific echocardiographic parameters, which could have been of interest. Moreover, we did not gather data on socioeconomic variables or treatment adherence, which could have an impact on incident HF.
CONCLUSIONSIn this study of older patients with AF and with no previous history of HF, the incidence of de novo HF was higher (11.5%) than previously described in younger population groups. Furthermore, the incidence was associated with poor prognosis (increasing the risk of mortality by 1.67-fold). Eleven clinical factors were related to the development of HF: significant valvular heart disease, reduced baseline LVEF, the presence of COPD, enlarged LA, renal dysfunction, malnutrition, anemia, permanent AF, diabetes, and higher age and BMI. Renin-angiotensin-aldosterone system inhibitors were a protective factor.
Further studies are needed to ascertain whether modifying these factors could decrease the risk of developing incident HF in older patients.
Atrial fibrillation is the most frequent arrhythmia in clinical practice, while heart failure is equally prevalent in the general population. Both entities increase with age and are considered the most significant cardiovascular epidemics. It is well known that HF and AF are interconnected as regards comorbidity and some of their risk factors.
However, there are few reports of the factors that may precipitate HF onset in AF patients, especially in the subgroup of older patients, who are at high risk. Modifiable risk factors for HF should be targeted rigorously in this population to reduce the risk of incident HF, which may be subsequently associated with an increased risk of death.
What does this study add?This is the first study to analyze AF in older patients without a prior history of HF. In these patients, the incidence rate of de novo HF was higher than previously described. Moreover, the presence of HF was directly related to poor clinical outcomes, such as bleeding or embolism, and mortality.
Eleven risk factors stratified the likelihood of developing HF during follow-up in older people, with no differences among the LVEF group. This is the first time that risk factors have been studied by age, allowing the detection of the older population at risk for developing de novo HF.
No funding.
AUTHORS’ CONTRIBUTIONSM. Melendo-Viu: methodology, format analysis, writing original draft, reviewing and editing, and visualization. S. Raposeiras-Roubín: conceptualization, methodology, software, format analysis, writing, reviewing and editing, visualization, supervision, project administration. E. Abu-Assi: conceptualization, software, format analysis, writing, reviewing and editing, visualization, supervision, project administration. D. Dobarro-Pérez: writing, reviewing and editing, visualization. M. Castro Cabeza: investigation, data curation. S. Fernández Fernández: investigation, data curation. L. Pérez Expósito: investigation, data curation. Sonia Blanco Prieto: data curation, format analysis. E. García: resources, visualization, supervision. A. Íñiguez Romo: resources, visualization, supervision.
These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
CONFLICTS OF INTERESTNo relevant relationships with industry.