The heart failure syndrome with its complex clinical manifestations is the result of a variety of different pathophysiologic mechanisms.1 In the last few decades the incidence of heart failure has become so common in the occidental environment that it is recognized as an epidemic, with an associated prognosis comparable to or worse than most malignancies.2-5 Fortunately, there have been significant improvements in therapy for heart failure, with the development of disease modifying drugs, including beta-blockers, ACE-inhibitors, ARBs, aldosterone inhibitors, and the use of implantable devices such as ICDs or cardiac resynchronization therapy, all having been shown to improve survival, ameliorate symptoms and decrease hospitalizations.1-7 Cardiac surgical techniques have likewise improved and provide newer methods for ventricular reconstruction, revascularization, and mitral valve repair. However, in the advanced failing heart, classified as end-stage or stage D by the American Heart Association/American College of Cardiology (AHA/ACC) staging system, the appropriate approach has not been always so clear.8 The obligatory use of inotropic drugs in some of these patients who present with profound depression of cardiac output has been linked frequently with middle or long term increase in mortality.1 For a select group of patients, cardiac transplantation provides a great achievement, with a high increase in quality of life and long term survival; the limitations of transplant include the need for a risky immunosuppressive therapy, high costs, shortage of donors, and certain medical contraindications.9,10 In spite of that, since the introduction of the calcineurin inhibitors, heart transplantation has become the single most effective treatment of end-stage heart disease. Unfortunately, transplant is the answer for only a small number of patients and an additional approach has been needed.9-11 Mechanical circulatory assist devices (MCADs), or more commonly called VADs (ventricular assist devices) have been deemed to occupy this space.11
What Is a VAD?
Mechanical circulatory assist devices are implantable pumps which provide hemodynamic support, generating additional flow in the refractory (acutely or chronically) failing heart, when medical treatment or IABP (intraaortic balloon pumps) are neither sufficient nor suitable. Since the first left-sided VAD (LVAD) was implanted in 1963, and the initiation of the USA artificial heart program sponsored by the National Heart, Lung and Blood Institute (NHLBI) in 1964, several devices have been developed.11
MCADs were initially used for postcardiotomy heart failure in the 1970's. In 1978, a LVAD was used briefly for the first time as bridge, or transition, to transplant. In the 1980s, the FDA (Food and Drug Administration of the USA) approved VADs to provide circulatory support to patients with severe heart failure, and in 1984 the first long term device was used as bridge to transplantation. In 1988 the first axial pump was introduced, and in the 1990s the concept of outpatient, long-term support had begun to be routinely practiced. In 1996, after a VAD was successfully removed in a patient whose cardiac function had improved substantially, the concept of a MCAD "bridge to recovery" became a goal in some patients. In 2001, the results of the REMATCH trial demonstrated the concept that VADs could serve as permanent treatment, the so-called destination therapy, as an alternative to medical therapy for the patient with a profoundly failing heart.11,12 This landmark study, published in the New England Journal of Medicine, randomized 129 patients ineligible for transplantation, all in New York Heart Association functional class IV, to either mechanical circulatory support or medical therapy. The trial's investigators reported that VADs provided greater that a 2-fold survival benefit over maximal medical treatment, in the most seriously ill patients ever studied in a trial. Most recently, encouraging new generation VAD designs have been developed and introduced in common daily practice and experimental protocols.13
As the size of the newer generation devices has decreased, they have been used much more commonly in children as well as in smaller adults.11,12,14 According to their placement, VADs can be divided into extracorporeal, where the primary pump is located outside the body (ie, Centrimag, ECMO, Thoratec) or intracorporeal (ie, Heart Mate I, Heart Mate II or Ventrassist). Further details about classification are given in other issues of Revista Española de Cardiología.15,16
MCADs are used most commonly to support the left ventricle (LVADs), the right ventricle (RVADs) or both (BiVADs or, alternatively the total artificial heart).17 According to the flow provided by the devices, they can be divided into pulsatile or continuous circulatory assist. Currently there is a trend to design smaller intracorporeal and more durable pumps, (second and third generation, like Heart Mate II and Ventrassist, respectively), that utilize continuous flow. The number of VADs implanted is growing every year.17
In Which Clinical Scenarios Are MCADs Used?
There are several strategies that are now acknowledged as appropriate to consider the use of MCADs18:
- Bridge to transplant: In this clinical situation, a VAD is used to sustain a patient who might otherwise succumb while awaiting a heart transplantation. Use of a MCAD has been shown to improve renal function or optimize pulmonary pressures in such patients, and allows the patient to be transplanted in better conditions.19 It is particularly useful in patients with certain blood groups, ie, group O, where longer waiting time for transplant is expected, in order to avoid systemic organ damage.
- Bridge to recovery: in certain cases, mainly after cardiotomy, MCADs have allowed adequate time for the repaired heart to recover while supporting the hemodynamic status of the patient.17,20 Sustained reversal of severe heart failure secondary to nonischemic cardiomyopathy has been reported in selected patients with the use of a LVAD and a specific pharmacologic regimen, including clembuterol.21
- Bridge to decision: sometimes it is very difficult to immediately judge a patient as ideal for transplant, when a lot of the information needed for evaluating the candidacy for transplant is missing (medical conditions, drugs abuse, social support). A VAD can be a solution to support the patient until the workup is completed. Another recent use is to implant a VAD in a prohibitively obese patient to allow weight loss to occur safely, after which heart transplant is undertaken.
- Destination or permanent replacement therapy: when the patient is ineligible for transplant (age, medical, social reasons). This indication has been increasing since the technological development has allowed the use of more durable devices, with less complications, and more experience with outpatient programs.11
Ultimately, these strategies are not absolute but may evolve over the course of the patient, that is, a patient may move from one implanting strategy to another. Thus, after a time supported with a VAD until improvement of their secondary organ dysfunction, their pulmonary hypertension or other comorbidities which previously precluded the transplant, a patient initially ineligible, can undergo the procedure successfully and more safely.18,22
What About Their Results?
Outcomes have dramatically improved in recent VADs patients, as a result of major advances in device design, patient selection, perioperative management, and a multidisciplinary approach.23 The REMATCH study showed a reduction of 48% in the risk of death from any cause in the group that received LVADs as compared with the medical treatment cohort (52% vs 25%, at 1 year; 23% vs 8%, at 2 years, respectively). The quality of life was significantly improved at 1 year in the device group, as well.11 Strikingly, these differences were among the largest ever found between 2 treatments for heart failure patients.
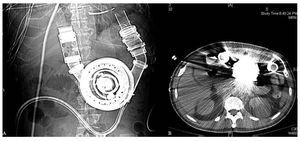
Subsequent to the REMATCH study, the design of the VAD used, a Heart Mate vented electric device was significantly improved, and additional devices have been commercialized (Figure). A recent study published by Miller using a new generation continuous-flow device (Heart Mate II) demonstrated a survival rate during support of 75% at 6 months and 68% at 12 months. At 3 months, therapy was associated with significant improvement in functional status and in quality of life.19
Figure. A and B: Heart Mate I. Intracorporeal pulsatile ventricular assist device. An improved design after the REMATCH trial. Although there are smaller pumps, this can be managed without anticoagulation.
The data reported by the University of Wisconsin, comparing the results of VADs implanted between 1990 and 2006 demonstrate these major improvements as well. Between 1990-1996 and the interval 2003-2006, the post VAD 1 and 3 year survival rates were 54.1% and 40.5% compared to 86.8% and 82.5%, respectively (P<.001).23 However, it should be noted that appropriate selection of candidates and early timing of VAD implantation is critical for improved results.24 Patients with advanced heart failure before major complications develop have the best chance of achieving an excellent 1 year survival with VAD therapy.24
What About the Complications?
Several complications derived from VAD implantation have been described.11-24 The following can be outlined: infection and sepsis, bleeding and tamponade, arrhythmias, neurological events and seizures, malfunction of the device, and right ventricular failure (with LVADs). Patients in the REMATCH device group were more than twice as likely as patients in the medical group to suffer a serious adverse event (rate ratio, 2.35; 95% confidence interval, 1.86-2.95). Sepsis was the most commonly reported cause of death (31%) in that study.11 Nevertheless, Osaki et al reported a decrease in the sepsis rate more recently (44.4% to 13.2%), in conjunction with better device design and an advanced antibiotic prophylaxis.23 The infection incidence rate has been estimated to be 5.9 infections per 1000 VAD support days at risk.25 In the recent post-REMATCH study by Miller et al, the most common adverse event was bleeding, mainly during the early postoperative period (31% requiring surgery, and 53% ≥2 units of red cells). Eight percent had strokes (mostly during the first 2 days after implantation).19
Localized infections not related to device were reported in 28% of patients, whereas device-related infections occurred in 14%. Sepsis was present in 20%. Respiratory failure was shown in 26%, ventricular arrhythmias appeared in 24%, right heart failure in 17%; non neurologic thromboembolic events in 7% and hemolysis in 3%. The most frequent causes of death during the first 180 days included sepsis, ischemic stroke, and multisystem organ failure.19 Probably the most important lesson learned from these early years of MCAD experience is the critical role of patient selection.24
In patients who have had VADs as a bridge to heart transplant, some patients have increased antibodies, but this does not increase the risk of either humoral or cellular rejection after transplantation.26 VAD support has shown post-transplantation outcomes comparable to those not requiring VAD.14
How Much Does a VAD Cost?
The MCAD devices and their management are expensive. The editorial accompanying the REMATCH trial stated "We know now that ventricular assist devices prolong life; we do not yet know for how long and at what cost."27 This affirmation is still in force. Although a lot of studies about cost have been published, the exact cost is very difficult to measure: different environments, different devices, reimbursements policies, bioethical issues, and changing outcomes all serve to confound the analysis. In 1995, Mehta et al considered that VAD patient had a superior rate of hospital discharge, at equitable daily cost.28 Miller et al, reported lower hospital costs, as well as improved outcomes and shorter average length of stay.29
A study performed with the Heart Mate VE, reported an overall mean cost for the initial implant-related hospitalization to be about 210 000 dollars. Implantation costs were higher in non-survivors compared with survivors. The study's conclusion was that the cost of long-term LVAD implantation is commensurate with other life saving organ transplantation procedures like liver transplantation. As an evolving technology, there are a number of opportunities for improvement that will reduce cost in the future.30 A British study concluded that in the event the price of the device would reduce to 40 000 pounds, the value of the survival could readily justify further trials of VADs as destination therapy.31
What Team Do We Need to Begin a Program?
A well trained multidisciplinary team is required to maintain a successful VAD program. A MCAD team would share many features with a heart transplant team, with cardiologists, surgeons, VAD coordinators, nurses, social workers, psychologist, dietitians, technical support, an appropriate intensive care unit, ward and office necessary.23 A clear plan to delineate appropriate patient inclusion criteria and patient education must be carefully considered as well.
Conclusion
In the present era, MCADs have evolved into appropriate treatment options for patients in advanced heart failure. VAD programs and VAD implantation have become widespread around the world, lead by the US, Japan, Australia and countries like Germany and the UK. Although costly, they are not more expensive than other approaches, like liver transplant, available in Spain. For VADs, the future is today.
Correspondence: Dra. M. Jessup,
Cardiovascular Division, University of Pennsylvania, Medical Director,
Heart Failure/Transplant Program, University of Pennsylvania Health System,
6 Penn Tower, 3400 Spruce Street, Philadelphia, PA 19014, United States
E-mail: Mariell.Jessup@uphs.upenn.edu