To the Editor:
Atrial septal defect (ASD) is a rare complication after cardiac surgery, usually related to atrial septostomy for valve repair or replacement. Repair has traditionally required new surgery, with greater risk for the patient. We describe the case of a patient with iatrogenic ASD after surgery for mitral valve repair, who was successfully treated by percutaneous implantation of an Amplatzer device guided by intravascular ultrasound (IVUS). Little information is available on this therapeutic approach in the scientific literature.1,2
A 62-year-old man with a history of smoking, hypertension, type 2 diabetes mellitus, hypercholesterolemia, and chronic renal failure was admitted for non-ST-elevation acute coronary syndrome. Coronary angiography showed severe disease in the left main coronary artery and triple-vessel disease, with severe mitral regurgitation and normal systolic function. The patient underwent myocardial revascularization with quadruple aortocoronary bypass (internal mammary to anterior descending artery; saphenous to middle branch, posterior descending artery, and posterolateral branch of right coronary artery) and mitral annuloplasty with prosthetic ring through a right atriotomy, and septostomy. Perioperative transesophageal echocardiography (TEE) showed no mitral regurgitation. The patient was discharged 13 days after the procedure.
One month after the surgery, the patient was readmitted for dyspnea at rest and palpitations of 3 days' duration. The electrocardiogram showed an uncommon atrial flutter, with ventricular response at 130 bpm. The chest x-ray revealed cardiomegaly with vascular redistribution and left pleural effusion. External cardioversion was performed and the patient recovered sinus rhythm.
Transthoracic echocardiogram (TTE) showed a nondilated left ventricle with moderately depressed overall systolic function. The mitral repair presented slightly elevated gradients (5 mm Hg) and minimal residual regurgitation. A systolic-diastolic shunt in the foramen ovale was observed on Doppler ultrasound. Following injection of agitated saline solution, considerable passage of bubbles to the left atrium was observed during the Valsalva maneuver. TEE confirmed an ASD of 7 mm, with left-to-right systolic-diastolic flow (estimated Qp/Qs of 2.6).
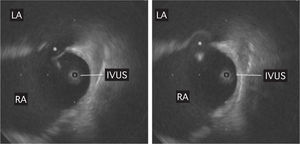
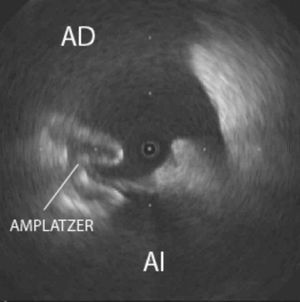
Percutaneous closure was proposed based on the diagnosis of postoperative ASD with significant left-to-right shunting. Bilateral femoral vein puncture using an IVUS catheter (9-Fr, 9-MHz Ultra ICE, Boston Scientific) confirmed the ASD in the fossa ovalis (maximum diameter 19 mm), and an image consistent with an interatrial septal aneurysm (Figure 1). A 20-mm Amplatzer ASD device (AGA Medical, Minneapolis, Minnesota) was successfully implanted (Figure 2). At 24 h, TTE showed that the device was correctly positioned and there was no residual shunt. The patient progressed favorably, with gradual improvement of the symptoms. Six months later, TTE showed no residual shunt. The patient was asymptomatic 1 year after surgery.
Figure 1. Intracardiac echocardiography image of the interatrial septum from the right atrium. The asterisk indicates the atrial septal defect at 2 time points during the cardiac cycle. The image of a possible aneurysm is also observed. IVUS indicates intravascular ultrasound; LA, left atrium; RA, right atrium.
Figure 2. Intravascular ultrasound image of the right atrium confirming correct positioning of the Amplatzer device anchored in the interatrial septum. LA indicates left atrium; RA, right atrium.
The usual surgical approach for mitral valve repair or replacement is left atriotomy. Alternatively, transeptal access from the right atrium can be performed, a particularly useful technique in nondilated left atria for optimal visualization of the mitral valve apparatus.3 However, this access is associated with a higher incidence of complications such as rhythm disorders (lesion to the sinus node artery or interatrial conduction pathways) and residual shunt through interatrial septal defects.4
The percutaneous approach with an Amplatzer device is widely used to repair congenital interatrial septal defects and is safe and effective, both at the short-term and mid- to long-term.5 Most teams choose the device size by measuring the defect with a catheter balloon and monitoring the procedure with TE.5 However, this implies the use of sedation or anesthesia with orotracheal intubation and requires the presence of an anesthetist and an echocardiography specialist in the interventional cardiology laboratory, lengthening the surgical procedure and increasing its complexity. Recent studies have reported that IVUS is useful for establishing the optimal size of the device, verifying correct placement, and confirming its final position, thus avoiding anesthesia and making the procedure more comfortable and less aggressive for the patient.6-8 The results obtained with this strategy have been described as excellent: greater accuracy in measuring the diameters of the defect (which TE tends to underestimate), shorter examination and surgical times, and minimal complications. The patients are discharged within 24 hours.6,7 In this case, a history of combined (valvular and coronary) cardiac surgery significantly increases the morbidity and mortality of a reoperation, thus making percutaneous implantation of an occluder device the strategy of choice.1,2
In conclusion, although ASD is a rare complication of cardiac surgery, IVUS-guided percutaneous closure with an Amplatzer appears to be a safe, effective alternative.