The PASCAL system is a novel device for transcatheter mitral valve repair based on the edge-to-edge concept. The unique features of this device might have a relevant impact on the repair outcomes. There are few data on clinical outcomes in real-life registries. The aim of this study was to report the early Iberian experience (Spain and Portugal) of the PASCAL system.
MethodsProcedural and 30-day outcomes were investigated in consecutive patients with symptomatic severe mitral regurgitation (MR) treated with the PASCAL system at 10 centers. Primary efficacy endpoints were technical success and degree of residual MR at discharge. The primary safety endpoint was the rate of major adverse events (MAE) at 30 days.
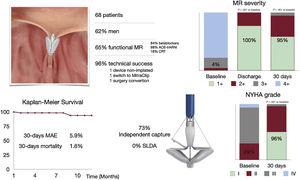
ResultsWe included 68 patients (age, 75 [68-81] years; 38% women; EuroSCORE II 4.5%). MR etiology was degenerative in 25%, functional in 65%, and mixed in 10%. A total of 71% of patients were in New York Heart Association (NYHA) functional class≥III. Technical success was achieved in 96% and independent capture was used in 73% of procedures. In the treated population, MR at discharge was≤2+ in 100%, with no in-hospital deaths. At 30 days, the MAE rate was 5.9%, the all-cause mortality rate was 1.6%, 98% were in NYHA functional class≤II, and 95% had MR≤2+ (P<.001).
ConclusionsTranscatheter mitral valve repair with the PASCAL system was safe and effective, with high procedural success and low rates of MAE. At 30 days, MR was significantly reduced, with a significant improvement in functional status.
Keywords
Mitral regurgitation (MR) is a highly prevalent and undertreated valvular heart disease, which carries a poor prognosis if left untreated.1–3 Transcatheter edge-to-edge repair (TEER) using the MitraClip system (Abbott Vascular, United States) has been consolidated as an effective therapy for high-risk patients with MR, with high procedural safety and producing rapid recovery.4–7 However, residual MR after TEER with MitraClip is not uncommon and is associated with worse outcomes.8
The PASCAL valve repair system (Edwards Lifesciences, United States) is a novel device for TEER. It offers special characteristic features including a central spacer, broad paddles made of pliable nitinol and the possibility of independent leaflet capture.9 PASCAL Ace is a new device designed with a narrow profile. Pivotal studies10 and German real-world experience11,12 demonstrated a low complication rate and high survival with sustained MR reduction. The present study assessed the real-world experience of a postapproval patient cohort treated at Spanish and Portuguese tertiary care centers.
METHODSStudy design and populationThis article covers the initial experience of transcatheter edge-to-edge mitral valve repair with the PASCAL system in the Iberian Peninsula (Spain and Portugal). This was a multicenter study collecting individual data from an unselected all-comer population from 10 hospitals with initial access to the PASCAL and PASCAL Ace systems for the treatment of severe symptomatic MR from October 2019 to September 2021.
Demographics, baseline and procedural characteristic and clinical/echocardiographic outcomes at follow-up were prospectively collected in a dedicated, shared database at each participating center. All procedures were performed under general anesthesia and guided by transesophageal echocardiogram (TEE) and fluoroscopy. Periprocedural complications were noted during the index hospitalization. The study was conducted in accordance with the institutional ethics committee of each participating center, and all patients provided signed informed consent for the procedure.
Endpoints and definitionsStandardized definitions of all patient-related variables, clinical diagnoses, and in-hospital complications and outcomes were based on the Mitral Valve Academic Research Consortium criteria unless indicated otherwise.13 MR was assessed using standard 2-dimensional color Doppler methods and were graded using a 4-class grading scheme: mild, moderate, moderate-severe, and severe.
The primary efficacy endpoints were technical success (device successfully deployed as intended, delivery system successfully retrieved without procedural mortality or need for surgery or reintervention) and severity of MR at discharge in the treated population.
The main safety endpoint was freedom from major adverse events (MAE) at 30 days, including all-cause mortality, cardiac tamponade, emergent surgery, vascular complications, severe bleeding (major, extensive, life-threatening, or fatal bleeding), stroke, and myocardial infarction.
Secondary endpoints included the severity of MR at discharge on an on-treatment basis (at least 1 PASCAL device implanted, alive and without reintervention), device success (at 30 days, defined as successful device implantation, MR≤2+, mean gradient <5mmHg and freedom from mortality, stroke, unplanned surgical or interventional procedures, and device failure), clinical success (as defined in the CLASP14 approval trial as successful device implantation, MR≤2+, and freedom from mortality, stroke, unplanned surgical or interventional procedures, and device failure at 30 days), reintervention, and change in clinical evaluation of New York Heart Association (NYHA) functional class during follow-up.
Statistical analysisResults are presented as mean±standard deviation for continuous normally distributed variables, median [interquartile range] for continuous nonnormally distributed data, and as number (percentages) for categorical data. Analysis of normality was performed with the Kolmogorov-Smirnov and Shapiro-Wilks test. Categorical data and proportions were compared using the chi-square or Fisher exact test. Comparisons of continuous variables were analyzed using the unpaired t-test and the Mann-Whitney U-test as appropriate. The McNemar paired-test and Wilcoxon matched-pairs signed-rank test were used to compare differences resulting from treatment as appropriate. An alpha error level of .05 was established and Stata 12.1 software (College Station, United States) was used for all analyses.
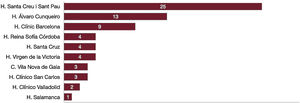
RESULTSPatient population and baseline characteristicsA total of 68 patients (median age 75 years, 62% men) with symptomatic MR underwent transcatheter mitral valve repair between October 2019 and November 2021. Baseline characteristics and center distribution are summarized in table 1 and figure 1. Patients were considered at high surgical risk, expressed by a mean EuroSCORE II of 4.5% and a relevant burden of comorbidities: 44% of patients had concomitant coronary artery disease, 13% had undergone surgical revascularization, and 7% had previous valve intervention. The prevalence of atrial fibrillation (59%) and chronic renal failure (32%) was high, most patients (71%) were in NYHA functional class III or IV and 38 patients (56%) had had a previous heart failure hospitalization in the last year.
Baseline characteristics
| Baseline characteristics | Total (n=68) | Functional MR (n=44) | Degenerative MR (n=17) |
|---|---|---|---|
| Age, y | 75 [68-81] | 72 [66-77] | 84 [76-87] |
| Women | 26 (38) | 18 (41) | 6 (35) |
| BSA | 1.79±0.18 | 1.79±0.17 | 1.76±0.20 |
| Hypertension | 57 (84) | 36 (82) | 15 (88) |
| Diabetes mellitus | 22 (32) | 17 (39) | 3 (18) |
| Atrial fibrillation | 40 (59) | 26 (59) | 10 (59) |
| CAD | 30 (44) | 19 (43) | 6 (35) |
| PCI | 22 (32) | 14 (32) | 5 (29) |
| CABG | 9 (13) | 4 (9) | 3 (18) |
| Stroke/TIA | 9 (13) | 2 (5) | 3 (18) |
| CKD | 22 (32) | 16 (36) | 5 (29) |
| Previous valvular intervention | 5 (7) | 2 (5) | 2 (12) |
| EuroSCORE II, mean | 4.5±3.6 | 4.6±3.9 | 3.5±2.6 |
| STS, mean | 4.1±3.0 | 4.0±3.4 | 4.2±1.9 |
| NYHA≥III | 48 (71) | 34 (77) | 9 (53) |
| Previous heart failure admission | 38 (56) | 28 (64) | 6 (35) |
| Medical treatment | |||
| Beta-blockers | 51 (75) | 37 (84) | 9 (53) |
| ACE-I /ARB | 35 (52) | 21 (48) | 10 (59) |
| Sacubitril/Valsartan | 24 (35) | 22 (50) | 0 (0) |
| Nitrates/Hidralazine | 10 (15) | 7 (16) | 0 (0) |
| CRT | 9 (13) | 8 (18) | 1 (6) |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blockers; BSA, body surface area; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; CRT, cardiac resynchronization therapy. NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons Score; TIA, transient ischemic attack.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Baseline echocardiographic parameters are shown in table 2. Most patients (96%) had moderate to severe (3+) or severe (4+) MR at baseline. MR etiology was classified as functional in 44 patients (65%), degenerative in 17 patients (25%), and mixed in the remaining 7 patients (10%). Median left ventricular ejection fraction was 43% and effective regurgitant orifice area 0.38 [0.30-0.49] cm2. Optimal guideline-directed medical therapy in secondary MR was achieved in a high number of patients: 84% of patients were treated with beta-blockers, 98% with angiotensin inhibition (angiotensin converting enzyme inhibitors/angiotensin II receptor blockers/angiotensin receptor-neprilysin inhibitors, [ACE-I/ARB/ARNI:]), and 18% were treated with cardiac resynchronization therapy (CRT).
Baseline echocardiographic variables
| Echocardiographic variables | Total (n=68) | Functional MR (n=44) | Degenerative MR (n=17) |
|---|---|---|---|
| LVEF | 43 [30-60] | 35 [26-55] | 60 [55-69] |
| LVEDD, mm | 59±9 | 62±9 | 52±5 |
| LVESD, mm | 46±12 | 50±12 | 37±6 |
| MR severity≥3+ | 65 (96) | 41 (93) | 17 (100) |
| MR mechanism | |||
| Functional | 44 (65) | ||
| Degenerative | 17 (25) | ||
| Mixed | 7 (10) | ||
| EROA, cm2 | 0.38 [0.30-0.49] | 0.39 [0.32-0.50] | 0.36 [0.28-0.55] |
| sPAP by echo, mmHg | 48±13 | 48±14 | 48±11 |
| Mitral valve area, cm2 | 5.4 [4.5-6.3] | 5.4 [4.4-6.2] | 5.5 [5.1-7.0] |
| Mitral mean gradient, mmHg | 1.7±0.9 | 1.8±1.0 | 1.6±0.7 |
EROA, effective regurgitant orifice area; LVEDD, left ventricle end-diastolic diameter; LVEF, left ventricle ejection fraction; LVESD, left ventricle end-systolic diameter; MR, mitral regurgitation; sPAP, systolic pressure pulmonary artery.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Procedural outcomes are summarized in table 3. One PASCAL device was implanted in 52 patients (76%) and 2 devices in 16 patients (24%). Overall, technical success was achieved in 65 patients (96%). The procedure was aborted without PASCAL implantation in 3 patients (4%). One patient with a short posterior leaflet had a tear with severe MR and underwent surgical repair. The second patient had leaflet tip calcification precluding appropriate PASCAL closure; the device was removed and a MitraClip was subsequently implanted. The third patient had worsening MR after leaflet clasping due to mitral valve distortion and the device was ultimately not released. Independent clasp optimization was applied in 47 patients (73%). Median procedural time was 121 [IQR: 83-168] minutes. There were no cases of intraprocedural single leaflet device attachment. Median length of stay after the procedure was 2 IQR: 1-3] days. There were no in-hospital deaths. In the treated population, residual MR at discharge was≤2+ in 100% of the patients and≤1+ in 77%. The mean transmitral gradient increased from 1.7mmHg at baseline to 2.7mmHg at discharge (P = .0001). Transmitral gradient was significantly lower in patients treated with a single device than in those requiring 2 devices (2.4 vs 3.5mmHg, P=.0037). A mean gradient≥5mmHg was observed in 10% of patients (n=6) and was not related to the number of devices (3 patients treated with 1 device, P=.27) nor to the type of device (4 patients treated with PASCAL, 1 treated with ACE, and 1 treated with both devices, P=.10).
Procedural and in-hospital data
| Procedural data | Total (n=68) |
|---|---|
| Number of PASCAL per patient | |
| 1 | 52 (76) |
| 2 | 16 (24) |
| Type of PASCAL Ace/P10Ace | |
| Ace | 22 (33) |
| P10 | 43 (64) |
| Both | 2 (3) |
| Optimization | 47 (73) |
| Technical success | 65 (96) |
| Technical complications | |
| Partial detachment | 0 |
| Total detachment | 0 |
| Leaflet tear | 1 (1) |
| Chordae rupture | 0 |
| MitraClip switch | 1 (1) |
| Major adverse events | |
| Death | 0 |
| Cardiac tamponade | 0 |
| Emergent surgery | 1 (1) |
| Vascular complications | 0 |
| Major bleeding | 0 |
| Stroke | 2 (3) |
| Myocardial infarction | 0 |
| MR severity≤2+ at discharge | 65±100 |
| Mean gradient (mmHg) at discharge | 2.7±1.3 |
| Admission days | 2 [1-3] |
MR, mitral regurgitation.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
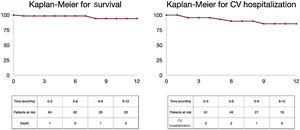
Thirty-day survival status was available in all patients (median follow-up time 223 [IQR: 105-439] days). Clinical success was achieved in 86.7% (59 of 68 patients). The primary safety endpoint, defined as a freedom from composite MAE rate at 30 days, occurred in 64 of 68 patients (94%). One patient died from cardiovascular causes due to sudden death at 30 days and another patient died from cardiovascular causes at 12 months of follow-up. The Kaplan-Meier survival curve of freedom from mortality is shown in figure 2. In-hospital stroke occurred in 2 patients and 1 patient had surgical conversion due to leaflet tear. At 30 days, 96% of patients were in NYHA class I or II and 95% of patients had MR≤2+, with significant clinical and echocardiographic improvement from baseline (P=.001, figure 3).
Central Illustration. Iberian experience with PASCAL for transcatheter mitral regurgitation repair. Baseline characteristics, procedural results, and follow-up. ACE-I/ARB/ARNI, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers/angiotensin receptor-neprilysin inhibitors; CRT, cardiac resynchronization therapy; MAE, major adverse events; MR, mitral regurgitation; NYHA, New York Heart Association; SLDA, single leaflet device attachment.
At 12 months, 5 patients were hospitalized for cardiovascular causes with a Kaplan-Meier curve for survival and cardiovascular hospitalization of 13.85% (figure 2).
DISCUSSIONWe report the first Iberian experience of TEER with the PASCAL device in patients with symptomatic functional and degenerative MR. The main findings of the study are: a) technical success was achieved in 96%, and MR in the treated population was reduced to grade≤2+ at discharge in 100% and to grade≤1+ in 77% of patients; b) all-cause mortality and the MAE rate at 30 days were low at 1.6% and 5.9%, respectively; and c) the main reasons for technical failure were aborted procedures or a change to the MitraClip device due to worsening of MR (n=2) or posterior leaflet tear (n=1).
Our findings are derived from unselected real-world patients. In this initial experience, the suitability criteria for PASCAL were similar as previously used for MitraClip therapy.
Some special features differentiate the MitraClip and PASCAL devices:
- -
The central spacer seems to generate less leaflet tension in the mitral valve, and it could be useful in MR with atrial or ventricular tethering.
- -
Independent capture of leaflets. The independent capture allows 1 leaflet to be released while the other is captured. Until the development of the PASCAL system, capture of leaflets had been simultaneous and when trying to optimize the capture of one there was a risk of losing the other. The new G4 MitraClip system also incorporates this special feature. However, differences between the aggressivity of leaflet capture in the MitraClip system can reduce its use, reported in 49% of its initial experience.15
Baseline population characteristics were comparable with other real-world data from the German registry,11,12 rather than the pivotal CLASP trial,10,14 targeting a highly comorbid population with a mean EuroSCORE II of 4.5% and advanced NYHA functional class,> 70% in class III-IV.
Our results are in line with previous efficacy endpoints reported with the PASCAL repair system. The CLASP study achieved 95% technical success with MR≤2+ in 96% of the patients at 30 days and real-world data from the German registry showed a technical success of 96% with residual MR≤2+ in 93% of the patients. The results of this experience confirmed the safety profile of the PASCAL Repair system, with zero procedural mortality and a low rate of 30-day MAE (5.9%), similar to that reported in the CLASP and German studies (8.3% and 4.1%, respectively). Of note, there were no cases of intraprocedural single leaflet device attachment (1% in the German registry), which may be due to a higher rate of independent clasp optimization (after initial simultaneous clasping) in the present series (73% vs 48% in the German study).
Importantly, there was a significant NYHA functional class improvement at 30 days (96% NYHA I-II), which is much higher than that observed in the German registry (72%), CLASP study (81%), or in our first reported experience with TEER with MitraClip in 201416 (81%).
Learning effectSince our first publication, the learning curve achieved with MitraClip in terms of patient selection and increased procedural experience may have strongly impacted on the positive results obtained with PASCAL in the present study. Indeed, patient selection has changed after the COAPT and Mitra-FR trials, which showed that patients with severe secondary MR with EROA> 0.3cm2 and LVESD <70mm under optimal medical treatment were more likely to benefit from TEER. Our data confirm these findings, with a median EROA value of 0.39cm2 [IQR: 0.32-0.50cm2], a mean LVESD of 50mm and optimal guideline-directed medical therapy: 84% patients with beta-blockers, 98% with ACE-I/ARB/ARNI, and 18% with cardiac resynchronization therapy.
In addition, increased procedural skills over time have led to a significant reduction in transseptal puncture-related complications (0% vs 3% in our initial experience with MitraClip), as well as a lower ratio of devices required per patient (≥ 2 devices required in 39% in MitraClip early-generation vs 24% in the present study).
LimitationsThe present study was observational in nature. Because the study was designed as a registry, all echocardiographic and clinical data were site reported and lacked independent adjudication by a core laboratory or an event committee. The absence of a randomized control group limits conclusions and the results for efficacy and safety should be regarded in the context of the early phase of the learning curve with a new device.
CONCLUSIONSTranscatheter mitral valve repair with the PASCAL device was safe and effective for treating severe MR, with a high technical success rate, a low rate of procedural complications, and sustained reduction in MR severity at 30 days. Larger studies with a longer-term follow-up are warranted.
FUNDINGThere was no funding.
AUTHORS’ CONTRIBUTIONSC.-H.P. Li: concept/design, data collection, data analysis/interpretation, drafting article, critical revision of article, approval of article, statistics. R. Estevez-Loureiro: concept/design, data collection, critical revision of article, approval of article. X. Freixa: data collection, critical revision of article, approval of article. R. Teles: data collection, critical revision of article, approval of article. A. I. Molina-Ramos: data collection, approval of article. M. Pan: data collection, critical revision of article, approval of article. L. Nombela: data collection, critical revision of article, approval of article. B. Melica: data collection, critical revision of article, approval of article. I. J. Amat-Santos: data collection, critical revision of article, approval of article. I. Cruz-González: data collection, critical revision of article, approval of article. Ll. Asmarats: doncept/design, data analysis/interpretation, critical revision of article, approval of article, statistics. R. Alarcón: data collection, critical revision of article, approval of article. L. Sanchis: data collection, critical revision of article, approval of article.
E. Fernández-Peregrina: data collection, critical revision of article, approval of article. J. A. Baz: data collection, critical revision of article, approval of article. X. Millán: concept/design, data analysis/interpretation, critical revision of article, approval of article, statistics. I. Menduiña: data collection, critical revision of article, approval of article. D. Arzamendi: concept/design, data analysis/interpretation, critical revision of article, approval of article.
CONFLICTS OF INTERESTC.-H.P. Li reports personal fees from Edwards and personal fees from Abbott, outside the submitted work. R. Estevez-Loureiro reports personal fees from Edwards, personal fees from Abbott, personal fees from Boston, outside the submitted work. L. Nombela has received consultant fees from Edwards Lifesciences and he is proctor for Abbott Vascular. L. Sanchis reports speaker honoraria from Abbott, Menarini, GE; she is proctor for MitraClip/TriClip implantation (Abbott) and associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. I. Cruz-González reports consultant fees for Edwards. Dr Melica reports personal fees from Abbott and Edwards, outside the submitted work. D. Arzamendi reports proctoring fees from Edwards Lifesciences.
- -
The PASCAL system is safe and effective: the CLASP study achieved 95% technical success with MR≤2+ in 96% of the patients at 30 days and real-world data from the German registry showed technical success of 96% with residual MR≤2+ in 93% of the patients. MAE previously reported in CLASP and German studies were 8.3% and 4.1%, respectively.
- -
The PASCAL system allows independent capture of leaflets, and it was used in 48% of patients in the real-world data from the German registry.
- -
The Iberian experience confirms the safety and effectiveness of the Pascal system: technical success was 96%, and MR in the treated population was reduced to grade≤2+ at discharge in 100% and to grade≤1+ in 77% of the patients. All-cause mortality and the MAE rate at 30 days were low at 1.6% and 5.9%, respectively.
- -
The main reasons for technical failure were aborted procedures or a change to the MitraClip device due to worsening of MR (n=2) or posterior leaflet tear (n=1).
- -
The independent capture rate was 73%, which was higher than in previous publications.
- -
The Iberian patient profile is adjusted to the COAPT trial with a high rate of optimal medical treatment.