Because of advances in cardiac structural interventional procedures, imaging techniques are playing an increasingly important role. Imaging studies show sufficient anatomic detail of the heart structure to achieve an excellent outcome in interventional procedures.
Up to 98% of atrial septal defects at the ostium secundum can be closed successfully with a percutaneous procedure. Candidates for this type of procedure can be identified through a systematic assessment of atrial septum anatomy, locating and measuring the size and shape of all defects, their rims, and the degree and direction of shunting. Three dimensional echocardiography has significantly improved anatomic assessments and the end result itself. In the future, when combined with other imaging techniques such as cardiac computed tomography and fluoroscopy, 3-dimensional echocardiography will be particularly useful for procedure guidance.
Percutaneous closure of the left atrial appendage offers an alternative for treating patients with atrial fibrillation and contraindication for oral anticoagulants. In the future, the clinical focus may well turn to stroke prevention in selected patients. Percutaneous closure is effective and safe; device implantation is successful in 94% to 99% of procedures. However, the procedure requires an experienced cardiac structural interventional team. At present, 3-dimensional echocardiography is the most appropriate imaging technique to assess anatomy suitability, select device type and size, guide the procedure alongside fluoroscopy, and to follow-up the patient afterwards.
Keywords
Progress in technology and human skills has led to the development of percutaneous interventions. As a result of new devices and techniques, treatment can be provided to a growing number of patients with structural heart disease. With the emergence of these procedures over the past 20 years, it is even more important and necessary for cardiologists to understand the anatomic particulars of heart structures and their complex 3-dimensional (3D) geometry to help them make informed decisions about which device to use, how best to position and deploy it, and how to locate and measure paravalvular leakage.
Echocardiography plays a key role in achieving this goal. Fluoroscopy affords real-time catheter visualization and angiography guides structure location, but these procedures provide insufficient anatomic detail. In contrast, magnetic resonance imaging and computed tomography (CT) provide the required anatomic detail but lack the benefit of real-time imaging. Three-dimensional transesophageal echocardiography (TEE) provides real-time images as well as unprecedented quality images and has therefore become the technique of choice in structural heart disease interventions. This technique has the unique ability to make heart structures look realistic and show anatomy in excellent detail, while visualizing the whole target site. These benefits significantly reduce intervention times.1–4
A new system has recently been developed that fuses fluoroscopic images and 3D TEE (EchoNavigator), serving to guide procedures with real-time images. The X-ray C-arm movement synchronizes with the 3D TEE, and thus the echo and X-ray images move in combination.
Intracardiac echocardiography, an auxiliary imaging technique for complex electrophysiology procedures, offers the key benefit of avoiding anesthesia and sedation. Another advantage is that it provides images of heart structures adjacent to the catheter with a similar or better quality than images obtained through TEE, without its limitations.5,6 Disadvantages include the high catheter cost, the need for specific user training, and, during the procedure, a second venous sheath. Also, intracardiac echocardiography carries a risk of inducing atrial arrhythmias and pericardial effusion. To date, intracardiac echocardiography has offered 2D imaging. The resulting slice makes it impossible to assess the true anatomic extent of a septal defect in a 3D context. Use of 3D techniques in intracardiac echocardiography is a promising novel technology.7
Cardiac structural intervention is a new procedure that requires a multidisciplinary approach, careful patient selection, and a thorough knowledge of the different devices involved. In addition, assessment of candidates for percutaneous interventions using imaging techniques differs from the assessment performed in other patients.
ATRIAL SEPTAL DEFECTAtrial septal defects (ASDs) account for approximately 6% to 10% of congenital heart defects at birth. In adulthood, ASD is the most commonly observed form of congenital heart disease, with a prevalence of 30% to 40% and predominance in women.
Atrial Septal Defect Anatomy and Associated AbnormalitiesPatent Foramen OvaleA patent foramen ovale (PFO) is actually a potential space or separation between the septum secundum and septum primum located in an anterior-superior position, rather than an atrial septal defect as such. This condition has a normal atrial septal tissue structure, which is why it is not classified as a true septal defect. The prevalence of a PFO in adults is 20% to 25%.
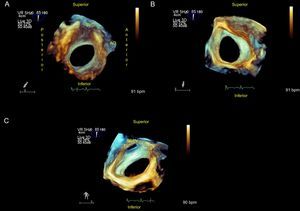
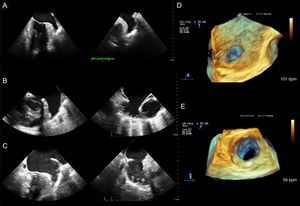
Ostium Secundum Atrial Septal DefectOstium secundum is the most frequent form of ASD (80%) and is located in the center of the septum. This presentation is the only ASD that is amenable to percutaneous closure (Figure 1 and Figure 2).
Ostium secundum atrial septal defect. A: View from the right atrium; device rims and spatial relationship with neighboring structures can be measured. B: From left atrium. C: Rotating the image, the connection from the right superior pulmonary vein to the left atrium can be observed. AO, aorta; SVC, superior vena cava; RSPV, right superior pulmonary vein.
Ostium secundum ASDs vary in shape (oval, round, or irregular), size (from a few millimeters to > 3 cm), and number (single or multiple) (Figure 1, Figure 2, and Figure 3). These anatomic variations may have significant implications in defect closure technique and may require the use of a device designed specifically for multiple ASDs or dictate the need for several closure devices. Ostium secundum defects rarely involve the vena cavae, atrioventricular valves, pulmonary veins or coronary sinus, but the cardiologist still needs to be know how close the defect is to these structures when considering percutaneous closure.
Atrial Septal AneurysmAtrial septal aneurysm is a sac-like redundancy or atrial septal deformity associated with increased mobility. This aneurysm is defined as a protrusion of septal tissue (typically of the fossa ovalis) measuring > 10 mm from the atrial septal plane in the direction of the right or left atrium, or a combined total protrusion from one side to the other measuring ≥ 15 mm. The prevalence of atrial septal aneurysm is 2% to 3% of the population and has been associated with the presence of a PFO and an increased prevalence of cryptogenic stroke and other embolic events. Atrial septal aneurysm has also been associated with multiple ASDs.8,9
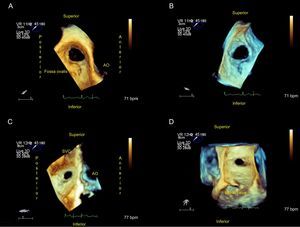
Types of Devices Used in Atrial Septal Defect ClosureThe indications and contraindications for percutaneous ASD closure are summarized in the Table. In general, devices are made of a nickel and titanium alloy called nitinol.10–12 Nitinol is a highly elastic metal with excellent shape memory (Figure 4).
Indications and Contraindications for Percutaneous Atrial Septal Defect Closure
| Indications • Ostium secundum ASD with pulmonary/systemic flow ratio (Qp/Qs) > 1.5 • Signs of right ventricular volume overload |
| Contraindications (absolute or relative) • Small ASD with Qp/Qs < 1.5 or without signs of right ventricular volume overload • A single defect too large for closure (> 38 mm) • Multiple defects unsuitable for percutaneous closure • Defect too close to superior or inferior vena cava, pulmonary veins, atrioventricular valves or coronary sinus • Anterior, posterior, superior or inferior rim < 5 mm. A retroaortic rim of < 5 mm is common and is not a contraindication for percutaneous closure if the other defect rims are present • Anomalous pulmonary venous drainage • Associated congenital anomalies requiring cardiac surgery • ASD with severe pulmonary arterial hypertension and bidirectional or right-to-left shunting • Nickel allergy |
ASD, atrial septal defect.
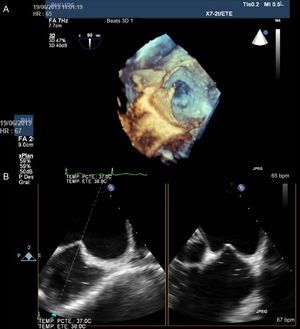
The Amplatzer ASD device is the most commonly-used device. The 3 elements in this device are a left disc, waist, and right disc, which is filled with polyester to facilitate thrombosis and total occlusion. The device size ranges from 4 mm to 40 mm and it should be selected by the waist diameter that fits the defect edges. An extra 1 mm or 2 mm should be added to the maximum diameter found in the defect (Figure 3 and Figure 4). The 2 flat discs spread out from the central waist to anchor the device securely.
Patients with ostium secundum ASD who are candidates for percutaneous closure usually have a left-right shunt, which is why the left atrial disc is larger than the right one.
The length of the atrial septum should be measured with a 4-chamber view because it must be greater than the disc diameters. This consideration is particularly important in children.
Occlutech Figulla Flex IISimilar to the Amplatzer, the third generation Occlutech consists of 2 discs connected by a thick waist. The Occlutech has reduced metal content and softer edges. Defect size must be carefully measured to avoid any residual shunt from underestimation.
Nit-Occlud ASD-RThe Nit-Occlud ASD-R consists of 2 discs knitted from a single nitinol wire mesh without soldering or fixation clamps, and a short connecting waist. This device has a significantly reduced metal content: 50% less than the Amplatzer device. Both discs have the same diameter. The device size should be the same size as the defect or 1 mm to 2 mm larger.
Preintervention AssessmentTransthoracic echocardiography is used for the initial assessment. The most relevant views are the long-axis and short-axis parasternal views from the subxiphoid window, the apical 4-chamber view, and the parasternal short-axis view.
In transesophageal echocardiography, through a midesophageal view, a sweep must be performed from 0° to 150° (4-chamber plane: 0°, 15°, 30°; aortic short-axis plane: 45°, 60°, 75°; vena cavae plane: 90°, 105°, 120°; long-axis plane: 135°, 150°). Three-dimensional TEE provides a direct image of the defect and information about many of its characteristics.
The operator must assess specific characteristics of the ASD (Figure 1, Figure 2 and Figure 3). First, as described in the , the following parameters are assessed: ASD type (ostium secundum, ostium primum, (venosus or coronary sinus, presence of a PFO, anomalous pulmonary vein drainage),13–18 size (maximum and minimum diameters and area), shape (round, oval, or irregular), the presence of multiple fenestrations, and its location in the atrial septum.
Second, color Doppler flow direction is assessed: left to right, right to left, or bidirectional flow, and the presence or absence of an atrial septal aneurysm, Eustachian valve or Chiari network, and their characteristics. All rims of the defect must be measured: the superior vena cava rim, superior rim between the ASD and the superior vena cava; the aortic or anterior-superior rim between the ASD and the AV valve; the inferior vena cava or posterior-inferior rim between the ASD and the inferior vena cava; the posterior rim between the posterior rim of the ASD and the posterior atrial walls; and the right upper pulmonary vein rim between the posterior rim of the ASD and the right upper pulmonary vein. Care should be taken to identify the presence of the posterior and inferior rims and to assess the dynamic nature of the defect (size variations during the cardiac cycle).
Periprocedural MonitoringThe size of the ASD is measured again during the closure procedure. The defect diameters can be directly measured with 3D TEE, which permits an en face view. If there is any doubt, a stop-flow diameter can be measured by inflating the balloon until the color flow across the defect has completely ceased. The diameter of the balloon within the atrial septum is measured in several imaging planes.
The selected device is then advanced through the orifice in a sheath connected to a guide wire. The left atrial disc is deployed, and after achieving correct apposition with the atrial wall, the right atrial disc is then deployed. Before the device is released, a complete assessment is performed of the closure device, atrial septum and surrounding structures. The cardiologist must try to identify the presence of atrial septal tissue between the left and right device discs. A slight residual shunt is a fairly common observation.
Assessment of the Final ResultAfter releasing the device, the cardiologist must carry out a complete assessment to detect any alterations in valves or veins, and any residual peripheral shunts. A follow-up ultrasound should be performed in all patients before discharge, again at 1, 6, and 12 months postprocedure, and every 1-2 years thereafter.
Acute and Chronic Complications of Percutaneous Closure19- •
Cardiac perforation causing pericardial effusion and tamponade
- •
Device embolization requiring percutaneous or surgical removal
- •
Hemorrhage
- •
Pulmonary embolism
- •
Device thrombosis
- •
Infectious endocarditis
- •
Device fracture
- •
Erosion. Erosion is a very rare complication. Risk factors for erosion reported with the Amplatzer device include20–22:
- –
Aortic rim deficiency in several views.
- –
Superior rim deficiency in multiple views.
- –
High location of an ostium secundum atrial septal defect.
- –
Large-sized closure device measuring > 26 mm.
- –
Poor alignment between the discs and the defect.
- –
Tenting of the atrial free wall after device implantation.
- –
Pericardial effusion after device implantation.
- –
Atrial fibrillation (AF) is the most common cardiac arrhythmia. The incidence of AF is closely associated with age and it affects 3% to 5% of adults older than 65 years. The prevalence will increase gradually in coming years as a result of population aging. In Spain, the number of people with AF is currently estimated at 1 million.
Many pathologies are associated with AF. Nonvalvular AF causes 15% to 20% of cardioembolic ischemic strokes, which are probably the most serious complication of AF because of their high morbidity and mortality. Most emboli originate from thrombi formed in the left atrial appendage (LAA) and this is the case in up to 90% of patients with nonvalvular AF. The CHA2DS2-VASc index is recommended in stroke risk assessment. The index assesses the following parameters: congestive heart failure, hypertension, age ≥ 75 years (score doubled), diabetes mellitus, prior stroke or transient ischemic attack (score doubled), vascular disease, age between 65 and 74 years and female sex.23
Long-term oral anticoagulant (OAC) therapy is recommended in patients with nonvalvular AF and CHA2DS2-VASc ≥ 2. However, OACs entail an increased bleeding risk and they have other problems such as the need for regular monitoring, food and drug interactions, and unpredictable action in some cases. Bleeding risk can be assessed with the HAS-BLED score (hypertension, abnormal renal/liver function, stroke, bleeding history, labile INR, elderly, drugs/alcohol concomitantly). Therefore, the benefit-risk balance must be assessed on a patient-by-patient basis. Of the patients with an indication for OACs, only 30% to 50% receive this therapy.
Novel OACs that have been developed in recent years and tested in large-scale clinical trials have shown varied but significant bleeding rates, particularly in the gastrointestinal tract.
Surgical ligation of the LAA has also shown varied results, attributed to the difficulty of achieving complete appendage closure. In the past, ligation was often only incompletely achieved and as a result, concerns have increased regarding the safety of stopping anticoagulation therapy.
In recent years, percutaneous occlusion of the LAA has been developed as an alternative to OAC therapy, especially among patients at high cardioembolic risk in whom OAC is contraindicated or inadvisable.
Devices Used in Left Atrial Appendage OcclusionAt present, the 2 most widely-used devices are the Amplatzer-Amulet system (St. Jude) and the Watchman system (Boston Scientific).
Amplatzer-Amulet Device, a Second Generation Amplatzer Cardiac PlugThe device has a circular design and is manufactured in a highly flexible nitinol braid. A lobe connects to a disc by a waist that allows for highly flexible articulation and adaptation prior to device deployment. The lobe adapts to the internal wall of the LAA and its hooks or stabilizing wires grasp the wall to ensure anchorage. The disc is designed to cover the LAA ostium and should be firmly wedged in and drawn outwards by the lobe. With the Amplatzer-Amulet system, the lobe is deployed 10 mm distally from the ostium and the disc should completely cover the LAA.
Watchman DeviceThis umbrella-shaped LAA occlusion device consists of a nitinol frame covered with a polyethylene membrane and fixation barbs. Since the Watchman device has no disc, there is no interference with the pulmonary veins or mitral valve. The device is released 10 mm from the LAA ostium and the orifice itself remains uncovered.
Both the Watchman and Amulet devices are deployed transeptally using a femoral approach. Both devices are highly flexible and are equipped with a set of stabilizing wires that grasp the LAA wall to prevent embolization. These LAA closure devices are available in clinical practice throughout Europe.
Two randomized controlled trials and several noninterventional studies have collected data from more than 2400 patients with nonvalvular AF who received a Watchman device to reduce stroke risk.
The PROTECT AF study is the only randomized trial that has compared warfarin therapy and Watchman device LAA closure in patients with nonvalvular AF. The study found that, at the 4-year follow up, LAA closure was superior to warfarin in the primary composite endpoint of stroke, systemic embolism, and cardiovascular or unexplained death, but at the start of the study, a worrying rate of periprocedural events was observed, with a 4.4% incidence of severe pericardial effusion.24–26
The procedure has a marked learning curve with an elevated risk of pericardial effusion among inexperienced operators.
The Watchman LAA occlusion device was approved by the US Food and Drug Administration very recently, in March 2015.27 The Amplatzer Cardiac Plug device was first used in 2008. Several retrospective studies have evaluated the device, mainly in Europe, Canada, Asia and Latin America, and have found a good safety profile and a successful procedure outcome.28–33 The current Amulet device has been modified to facilitate deployment and increase fixation. The device was relaunched in a restricted setting in October 2014.34-36
Current IndicationsIn real-world practice, LAA closure procedures are indicated mostly in anticoagulated patients referred by neurology services after an intracranial hemorrhage or by gastrointestinal services after finding recurrent gastrointestinal bleeding of unknown cause. Other situations that may justify LAA percutaneous closure are labile INR and cardioembolic events despite OAC therapy and controlled INR. In any event, the decision to opt for this procedure should be made by a multidisciplinary team that will assess not only the individual patient's cardioembolic and bleeding risk but also important factors such as treatment effectiveness, patient frailty and medication compliance, which is especially important in OAC therapy. Closure of the LAA protects against embolism and permits most patients to discontinue OAC therapy for life.
European Society of Cardiology Recommendations for Left Atrial Appendage Closure (2012)Percutaneous closure of the LAA may be considered in patients at high risk of stroke and contraindications to long-term OAC therapy (class IIb, level of evidence B).37-39
This recommendation is based on studies on the Watchman device and patients without contraindication to anticoagulation. Considering that LAA occlusion techniques have progressed and the Watchman device has received Food and Drug Administration approval since this guideline was published, recommendations may be extended in future updates.
Preprocedural AssessmentIt is essential to have good anatomical knowledge of the left atrium, and LAA morphology in particular before a patient is indicated for percutaneous closure of the LAA.40-41
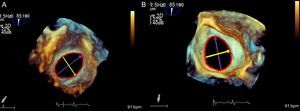
The LAA is characterized by its variability (Figure 5). The LAA can be divided anatomically into 3 regions: the ostium, neck, and lobar region. The ostium and neck are like a spiral staircase. The LAA varies in size from small to large. A large LAA is found in chronic AF, when its volume can be 3 times larger than normal. It is usually oval shaped, with marked differences between the minimum and maximum diameters, and sometimes it is circular. The diameters change significantly during the cardiac cycle when the heart is in sinus rhythm, and minimally in AF. Diameter values may also change in the presence of volume overload. The operator should count the number of lobes and analyze their arrangement and spatial relationship.
In 2D TEE, the transducer array is rotated through several midesophageal planes, at 0°, 45°, 90°, and 135°, to obtain the minimum (45°-70°) and maximum ostium diameters (approximately 135°), and to reconstruct the LAA anatomy. These diameters vary significantly if the LAA is oval shaped and minimally if it is round.
Ostium diameters range from 10 mm to 40 mm; LAA length ranges from 16 mm to 51 mm (mean, 31 mm), and volume from 0.7 mL to 19.2 mL. Low flow velocities of < 20cm/s are usually found in the LAA and they show spontaneous echocardiographic contrast. The LAA may have 1, 2 or more lobes, with several inner membranes. The operator should also measure the distance between the LAA and the mitral annulus and between the LAA and the circumflex artery.
The LAA must be thrombus-free before percutaneous closure is performed, it must have adequate dimensions, a minimum length or depth, and sufficient width in the device deployment zone to fit currently available devices. Usually, the landing zone diameter is measured with TEE using the aorta short axis view at 45° at the edge of the LAA internal wall, proximally to the circumflex artery, almost inside the appendage, and up to the LAA roof, at least 10 mm below the ostium.
Multiplane 3D TEE imaging gives a simultaneous view of 2 orthogonal planes. Similar measurements can be taken using different planes (rotated through 0°, 45°, 90°, and 135°), particularly at a perpendicular plane to 45° (ie, at 135°), and the diameters at the device landing zone can be measured.
This technique affords a high quality image when viewing the LAA en face. The LAA may have 1, 2 or more lobes, with several inner membranes. Of all imaging techniques, TEE is indispensable when assessing deployment feasibility and taking anatomical measurements.
Some authors use CT for diagnostic purposes and to select device size.42,43 This technique provides spatial information to help optimize implantation strategy and select device size.
Recent studies with multislice CT have shown that this technique is useful, fast, and precise when detecting thrombi and that it is a feasible alternative to TEE. In addition, multislice CT permits a fast and complete assessment of LAA anatomy and dimensions prior to the closure procedure, and evaluation of the device position postprocedure.
Studies have been conducted using different imaging techniques (CT, fluoroscopy, and TEE) and the different measurements obtained for the landing zone have been compared by technique. The largest measurement is obtained with CT, followed by TEE, and finally with fluoroscopy.
Multidetector CT (MDCT) and cardiac magnetic resonance imaging have been used to study LAA morphology, and morphology type has been correlated with prior stroke and transient ischemic attack.44 Four morphological types to describe LAA are chicken wing (frequency, 48%9), cactus (30%), windsock (19%), and cauliflower (3%). Patients with chicken wing morphology are less likely to experience an embolic event. However, LAA closure has been reported to be more complex with this morphology type.45
In patients at high esophageal bleeding risk and contraindication to TEE, endoscopic ultrasound may be safer than other techniques to guide the LAA closure procedure, because complications are rare and are immediately detected during the procedure. Clinical trials have corroborated the use of endoscopic ultrasound in the presence of gastrointestinal bleeding in patients with esophageal varices, although this technique has not been described to specifically assess cardiac structures or to guide interventional cardiovascular procedures.46
Periprocedural GuidanceDuring the procedure, TEE is indispensable to verify correct positioning, device release, and confirmation of LAA occlusion. A 3D TEE-guided procedure is safer and easier to perform. The implantation technique consists of the following steps:
- 1.
Transseptal Puncture47
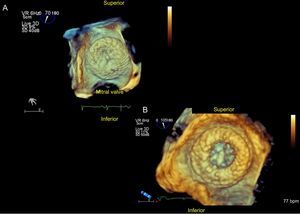
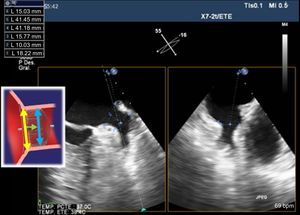
It is generally recommended to puncture the inferior-posterior zone of the fossa ovalis (Figure 6), avoiding a PFO. Monitoring with TEE verifies the needle position, transseptal puncture during septal tenting and entry into the LAA. In addition, the EchoNavigator system fuses TEE and X-ray images in real time, enhancing puncture guidance.48
- 2.
Catheterization of the Left Atrium and Left Atrial Appendage
The catheter is advanced through the LA to the LAA. This maneuver is generally fairly fast. Monitoring under TEE guidance helps verify correct positioning in the LAA. To avoid complications, it is extremely important for the operator to have a complete view of the intracardiac catheters and devices. Catheter maneuvering must be performed with the utmost care to avoid perforation of the fragile LAA wall, which could cause cardiac tamponade or other serious complications.
- 3.
Measurement of the Left Atrial Appendage and Device Selection
The imaging technique of reference throughout this procedure is TEE. Cardiac CT and MRI provide useful additional information. The minimum and maximum diameters for performing an occlusion procedure are 11 mm and 31 mm across the landing zone, where the Amplatzer-Amulet lobe is deployed. The LAA must have a minimum depth of 10 mm for device implantation (Figure 7).
The Amulet device should be about 2 mm larger that the LAA neck diameter. The device should be slightly larger than the TEE and angiographic measurements, to reduce the risk of residual leak without increasing the risk of LAA wall erosion and rupture. This extra size will permit improved occlusion with a well-fitted device lobe, while avoiding displacement or embolization. However, if the device is oversized, it will be overcompressed (making it pear-shaped) and could embolize or affect adjacent structures (mitral valve apparatus or circumflex artery). If the device is undersized, occlusion will not be achieved and it could also embolize or result in significant residual flow in the LAA. In the case of the Watchman device, the maximum diameter of the LAA ostium is measured and diameters between 17 mm and 31 mm are considered to be suitable.
- 4.
Device Deployment, Positioning, and Release
After the device lobe is deployed in the landing zone, the disc is released. The disc must adopt an umbrella shape and be wedged correctly in the LAA osteum (Figure 8).
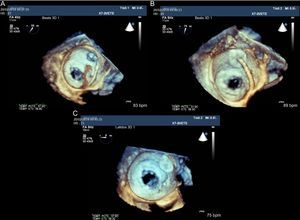
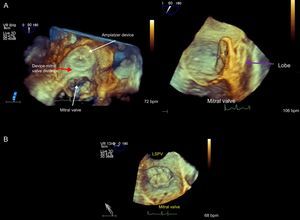
Figure 8.Three-dimensional transesophageal echocardiographic view from the superior portion of the left atrium at the entry to the left atrial appendage. The mitral valve is open. A: Delivery wire crossing the atrial septum and advancing towards the left atrial appendage. B: Amplatzer Cardiac Plug device positioned in the left atrial appendage prior to release. C: Device position after release in relation to the mitral valve.
(0.27MB).Device stability and position are verified with fluoroscopy and TEE by performing a tug test with the release wire. The device must be firmly in position, occluding the ostium, without permitting residual flow. After verifying these points, the device can be released. In addition, neighboring structures such as the mitral apparatus and the circumflex artery must receive no interference.
Imaging with 3D TEE is particularly useful to rule out device displacement after release and to reassess any device interference with neighboring structures.49
The lobe must be slightly compressed, adopting a car tire shape. The disc must be convex and point into the LAA (Figure 9). The color Doppler must show no flow into the LAA. The absence of thrombi must be confirmed (Figure 10). At the end of the procedure, echocardiography is used to assess the presence of any immediate complications:
- •
Pericardial effusion or tamponade.
- •
Device migration and embolization: device embolization is usually caused by inaccurate measurement of the LAA ostium or by device release problems.
- •
Incomplete occlusion of the LAA, increasing the risk of thrombosis and embolic events.49,50
- •
Interference with neighboring structures. It is important to confirm that the device has no impact on mitral flow or pulmonary vein flow and to rule out alterations in regional contractility secondary to circumflex artery compression.
- •
Residual ASD from the transseptal puncture.
Three-dimensional transesophageal echocardiographic view from the LA roof (A) and on a sagittal plane (B); note the distance between the Amplatzer Cardiac Plug device and the mitral valve (red arrow) and the device lobe position (purple arrow) inside the left atrial appendage. Below, Watchman device occluding the left atrial appendage. LSPV, left superior pulmonary vein.
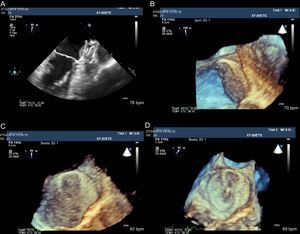
Two- and 3-dimensional transesophageal echocardiographic images of a thrombus located on the atrial side of the Amplatzer Cardiac Plug disc (A). B and C: Partial vision loss of the figure-of-8 shaped device mesh. D: After 2 months of low molecular weight heparin therapy, the figure-of-8 mesh image is recovered, indicating thrombus resolution.
A repeat echocardiographic study should be performed after the procedure and before the patient is discharged to confirm that the device is still well positioned and to rule out pericardial effusion. Follow-up studies should be repeated after 1, 3 or 6, and 12 months, and then once yearly.
CONFLICTS OF INTERESTA. Bethencourt González is a proctor for St. Jude Medical.