To determine whether structural/organizational characteristics of hospitals and emergency departments (EDs) affect acute heart failure (AHF) outcomes.
MethodsWe performed a secondary analysis of the EAHFE Registry. Six hospital/ED characteristics were collected and were related to 7 postindex events and postdischarge outcomes, adjusted by the period of patient inclusion, baseline patient characteristics, AHF episode features, and hospital and ED characteristics. The relationship between discharge directly from the ED (DDED) and outcomes was assessed, and interaction was analyzed according to the hospital/ED characteristics.
ResultsWe analyzed 17 974 AHF episodes included by 40 Spanish EDs. Prolonged stays were less frequent in high-technology hospitals and those with hospitalization at home and with high-inflow EDs, and were more frequent in hospitals with a heart failure unit (HFU) and an ED observation unit. In-hospital mortality was lower in high-technology hospitals (OR, 0.78; 95%CI, 0.65-0.94). Analysis of 30-day postdischarge outcomes showed that hospitals with a short-stay unit (SSU) had higher hospitalization rates (OR, 1.19; 95%CI, 1.02-1.38), high-inflow EDs had lower mortality (OR, 0.73; 95%CI, 0.56-0.96) and fewer combined events (OR, 0.87; 95%CI, 0.76-0.99), while hospitals with HFU had fewer ED reconsultations (OR, 0.83; 95%CI, 0.76-0.91), hospitalizations (OR, 0.85; 95%CI, 0.75-0.97), and combined events (OR, 0.84; 95%CI, 0.77-0.92). The higher the percentage of DDED, the fewer the prolonged stays. Among other interactions, we found that more frequent DDED was associated with more 30-day postdischarge reconsultations, hospitalizations and combined events in hospitals without SSUs, but not in hospitals with an SSU.
ConclusionsAHF outcomes were significantly affected by the structural/organizational characteristics of hospitals and EDs and their aggressiveness in ED management.
Keywords
Good treatment outcomes after acute heart failure (AHF) are largely dependent on the organizational structure surrounding patient care.1,2 Patient management organization is especially important in view of the failure of new treatment proposals for this syndrome3–5 and the essentially unaltered prognosis of AHF patients over the past several decades.6 Prominent among proposed organizational approaches is the transfer of care during decompensation episodes, and many Spanish hospitals have devised specific multidisciplinary protocols, many of them based in heart failure units (HFU).2,7
In addition to these specific intervention programs, AHF patient outcomes can also be affected by the inherent characteristics of the hospital and emergency department (ED). Key hospital characteristics include hospital type (high-technology or community) or whether the hospital has a short-stay unit (SSU), a home hospitalization (HH) service, or an HFU. Some hospitals are rapidly introducing the latest forms of telemedicine as a useful way to manage AHF patients during convalescence and even during the stabilization period between decompensations.8 The other key organizational element of patient care is the ED, since more than 90% of AHF patients are first treated there9 and 15%-35% of them are discharged from the ED without hospital admission.10 Patient outcomes can be affected by ED patient inflow, the availability of a specific observation unit for treatment-response monitoring, and the frequency of discharge directly from the ED (DDED) without hospital admission. Until now these organizational features have received very little attention in the Spanish health system. We hypothesized that all of these structural/organizational features will affect AHF outcomes and that each will have a specific outcome. The goal of the present study was thus to investigate the effect of these hospital and ED structural/organizational characteristics on AHF outcomes.
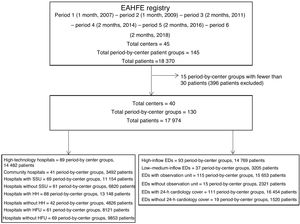
METHODSStudy designWe performed a secondary analysis of the EAHFE Registry (Epidemiology of Acute Heart Failure in Emergency departments). The EAHFE Registry is a prospective, multicenter, multipurpose, noninterventional analytical cohort study that to date has had 6 patient inclusion periods involving the participation of 45 Spanish EDs (representing 15% of public sector hospitals) and has included 18 370 patients.11,12
Here, we designed an exploratory study that, for each EAHFE Registry inclusion period, included those hospitals recruiting at least 30 patients. Hospitals with fewer than 30 recruited patients within a defined inclusion period were excluded to minimize the risk of nonconsecutive inclusion and to avoid distortion of outcome estimates due to low patient numbers. In each inclusion period, hospital and ED structural/organizational characteritics were considered independently, so that the same hospital could be assigned to one characteristic category in one inclusion period and to the opposite category in another if its situation in relation to that characteristic had changed.
Hospital and emergency department organizational characteristicsWe defined 4 hospital structural/organizational characteristics: a) hospital complexity (high-technology vs community); b) the presence of an SSU (a dedicated unit for the admission of patients with the specific aim of discharge without transfer of care within a prespecified maximum time frame, normally 72-96hours); c) the presence of an HH service (a specific program for the provision of hospital care at home until patient discharge, under the supervision of hospital physicians and nursing staff); and d) the presence of an HFU (a structured multidisciplinary unit with established protocols for the care and follow-up of selected HF patients). The following 3 ED characteristics were considered: a) patient inflow (high-inflow was defined as more than 300 daily consultations); b) the presence of an observation unit (a physical space within the ED separate from the primary care area and dedicated to complementary examinations and treatment assessments of patients under the supervision of ED medical staff, generally in a period shorter than 24hours; and c) the DDED rate in each inclusion period (an indirect index of the aggressiveness of AHF management in the ED, with a higher DDED rate indicating more aggressive management).
Patient characteristicsThe 40 independent variables fall into the following categories: demographic data (age and sex); comorbidities (hypertension, diabetes mellitus, ischemic heart disease, atrial fibrillation, valvular heart disease, previously diagnosed heart failure, cerebrovascular disease, peripheral artery disease, chronic kidney disease, chronic obstructive pulmonary disease, active cancer, and dementia); chronic heart failure treatments (loop diuretics, renin-angiotensin system antagonists, beta-blockers, mineralocorticoid receptor antagonists, and digoxin); baseline status during the month before the decompensation episode (general functional status according to the Barthel index, respiratory function according to the New York Heart Association [NYHA], and the left ventricular ejection fraction [LVEF]); decompensation triggers (infection, tachyarrythmia, anemia, hypertensive crisis, dietary or treatment nonadherence, and acute coronary syndrome); decompensation episode severity (stratification of risk on the MEESSI scale11,13 and the need for hospital admission); and treatment both in the ED (diuretics, nitrates, morphine, digoxin, inotropes/vasopressors, and noninvasive ventilation) and during hospitalization or after discharge (renin-angiotensin system antagonists, beta-blockers, mineralocorticoid receptor antagonists, and placement of a cardiac resynchronization pacemaker).
Outcome variablesA total of 7 outcomes were defined. Among them, 3 were related to the AHF index event: a) in-hospital mortality (before discharge after the index event); b) prolonged stay (> 10 days between the index event and discharge); and c) 30-day all-cause mortality. The other 4 variables were related to follow-up during the 30 days postdischarge and apply only to index event survivors: a) postdischarge ED reconsultation for AHF; b) postdischarge hospitalization for AHF; c) postdischarge all-cause mortality; and d) postdischarge combined event (death, ED reconsultation, or hospitalization).
Statistical analysisQualitative variables are expressed as frequencies and percentages, and quantitative variables are expressed as mean±standard deviation or median [interquartile range]. The relationship between structural/organizational characteristics and the outcome variables was analyzed by logistic regression; nonadjusted and adjusted odds ratios (OR) were calculated and are presented together with their 95% confidence intervals (95%CI). In the adjustment strategy, the following covariables were introduced in sequence for each outcome variable: inclusion period (year), the 40 patient characteristics considered in the analysis, and finally the center. When the analysis detected a center-dependent effect independent of the inclusion period and the patient characteristics, we removed the center variable from the model and explored which hospital or ED structural/organizational characteristics were related to the outcomes of interest; once identified, these characteristics were introduced into the model together with the other factors. Before adjusting the model, we used multiple imputation to create 10 new datasets with no missing values for any of the variables corresponding to patient characteristics. Multiple imputation analysis was performed with the SPSS statistical package, and pseudorandom numbers were generated with the Mersenne Twister algorithm, using the number 2 000 000 as seed.
To analyze the effect of the DDED rate on outcomes, we first calculated the percentage DDED for each ED in each inclusion period and then analyzed its relationship with the 7 outcome variables by linear regression analysis to calculate the determination coefficient (R2). For this analysis, each period-by-center patient group was treated as an individual unit independently of the number of patients it included. We also analyzed whether the DDED–outcome relationship interacted with the structural/organizational characteristics. For this analysis, we centered interaction variables on the mean to avoid the multilinearity problem that arises with noncentered variables. The centered variables were added to the principal effects model generated from the independent variables. The presence of collinearity was analyzed with the variance inflation factor.
The robustness of the adjusted outcomes in the principal analysis was assessed in 4 sensitivity analyses of statistically significant associations. Sensitivity analysis A included only patients hospitalized after the index event; analysis B included only patients with AHF confirmed by natriuretic peptides; analysis C imputed only those variables for which fewer than 20% of individuals had missing values; and analysis D included no multiple imputation. Interaction with LVEF was assessed by analyzing statistically significant associations according to whether LVEF was preserved (≥ 50%) or reduced (< 50%).
Statistical significance was assigned at P<.05 or if the OR 95%CI excluded the value 1. Since the purpose of this study was an introspective general assessment of the possible effects of center characteristics on AHF outcomes, the P values should be considered nominal and descriptive, and were not adjusted by multiple comparisons. Collinearity was excluded if the variance inflation factor was<10. The statistical analysis was performed with SPSS software, version 24 (IBM, United States).
Ethical considerationsThe EAHFE Registry complies with the Helsinki declaration, and all patients gave written informed consent. The study protocol was approved by the Clinical Research Ethics Committee of the Hospital Universitario Central de Asturias (referencias 49/2010, 69/2011, 166/13, 160/15, and 205/17).
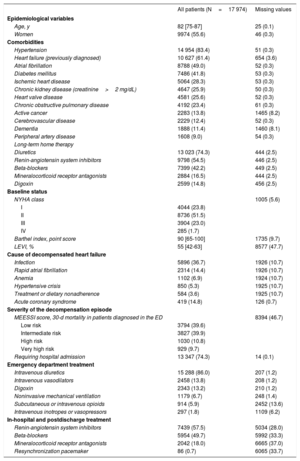
RESULTSOf the 18 370 AHF episodes in the EAHFE Registry, we analyzed 17 974 (97.8%) episodes recorded at 40 EDs, corresponding to 130 independent period-by-center groups (figure 1). Mean patient age was 82 years, 55.6% were women, and the sample presented many comorbidities. Other baseline and event characteristics are presented in table 1. Of the study population, 14 482 (80.6%) were treated in high-technology hospitals, 11 154 (62.1%) in hospitals with an SSU, 13 148 (73.2%) in hospitals with HH, 9853 (54.8%) at hospitals with an HFU, 14 482 (82.3%) at hospitals with a high-inflow ED, and 15 653 (87.1%) at hospitals with an ED observation unit.
Study population patient characteristics
| All patients (N=17 974) | Missing values | |
|---|---|---|
| Epidemiological variables | ||
| Age, y | 82 [75-87] | 25 (0.1) |
| Women | 9974 (55.6) | 46 (0.3) |
| Comorbidities | ||
| Hypertension | 14 954 (83.4) | 51 (0.3) |
| Heart failure (previously diagnosed) | 10 627 (61.4) | 654 (3.6) |
| Atrial fibrillation | 8788 (49.0) | 52 (0.3) |
| Diabetes mellitus | 7486 (41.8) | 53 (0.3) |
| Ischemic heart disease | 5064 (28.3) | 53 (0.3) |
| Chronic kidney disease (creatinine>2 mg/dL) | 4647 (25.9) | 50 (0.3) |
| Heart valve disease | 4581 (25.6) | 52 (0.3) |
| Chronic obstructive pulmonary disease | 4192 (23.4) | 61 (0.3) |
| Active cancer | 2283 (13.8) | 1465 (8.2) |
| Cerebrovascular disease | 2229 (12.4) | 52 (0.3) |
| Dementia | 1888 (11.4) | 1460 (8.1) |
| Peripheral artery disease | 1608 (9.0) | 54 (0.3) |
| Long-term home therapy | ||
| Diuretics | 13 023 (74.3) | 444 (2.5) |
| Renin-angiotensin system inhibitors | 9798 (54.5) | 446 (2.5) |
| Beta-blockers | 7399 (42.2) | 449 (2.5) |
| Mineralocorticoid receptor antagonists | 2884 (16.5) | 444 (2.5) |
| Digoxin | 2599 (14.8) | 456 (2.5) |
| Baseline status | ||
| NYHA class | 1005 (5.6) | |
| I | 4044 (23.8) | |
| II | 8736 (51.5) | |
| III | 3904 (23.0) | |
| IV | 285 (1.7) | |
| Barthel index, point score | 90 [65-100] | 1735 (9.7) |
| LEVI, % | 55 [42-63] | 8577 (47.7) |
| Cause of decompensated heart failure | ||
| Infection | 5896 (36.7) | 1926 (10.7) |
| Rapid atrial fibrillation | 2314 (14.4) | 1926 (10.7) |
| Anemia | 1102 (6.9) | 1924 (10.7) |
| Hypertensive crisis | 850 (5.3) | 1925 (10.7) |
| Treatment or dietary nonadherence | 584 (3.6) | 1925 (10.7) |
| Acute coronary syndrome | 419 (14.8) | 126 (0.7) |
| Severity of the decompensation episode | ||
| MEESSI score, 30-d mortality in patients diagnosed in the ED | 8394 (46.7) | |
| Low risk | 3794 (39.6) | |
| Intermediate risk | 3827 (39.9) | |
| High risk | 1030 (10.8) | |
| Very high risk | 929 (9.7) | |
| Requiring hospital admission | 13 347 (74.3) | 14 (0.1) |
| Emergency department treatment | ||
| Intravenous diuretics | 15 288 (86.0) | 207 (1.2) |
| Intravenous vasodilators | 2458 (13.8) | 208 (1.2) |
| Digoxin | 2343 (13.2) | 210 (1.2) |
| Noninvasive mechanical ventilation | 1179 (6.7) | 248 (1.4) |
| Subcutaneous or intravenous opioids | 914 (5.9) | 2452 (13.6) |
| Intravenous inotropes or vasopressors | 297 (1.8) | 1109 (6.2) |
| In-hospital and postdischarge treatment | ||
| Renin-angiotensin system inhibitors | 7439 (57.5) | 5034 (28.0) |
| Beta-blockers | 5954 (49.7) | 5992 (33.3) |
| Mineralocorticoid receptor antagonists | 2042 (18.0) | 6665 (37.0) |
| Resynchronization pacemaker | 86 (0.7) | 6065 (33.7) |
ED, emergency department; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Data are expressed as no. (%) or median [interquartile range].
In-hospital mortality was 7.4%, 22.4% of patients had prolonged stays, and 30-day all-cause mortality was 10.3%. Among the 16 546 index event survivors, the rates of AHF-related reconsultation, hospitalization, mortality, and the combined event were 29.5%, 20.7%, 5.0%, and 31.2%, respectively.
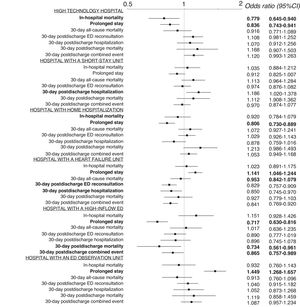
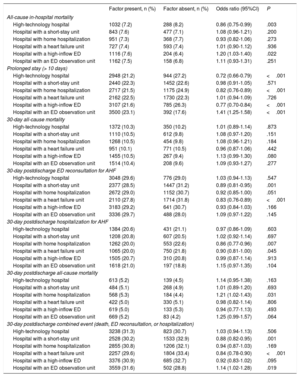
The univariate analysis showed a significant association between structural/organizational characteristics and some of the outcome measures (table 2). The progressive adjustment models revealed a center effect on all outcome measures (), and we therefore studied the effect of hospital and ED structural/organizational characteristics on all outcomes in a model adjusted for the inclusion period, the patient variables, and the structural/organizational characteristics. This analysis revealed the following results: high-technology hospitals had lower in-hospital mortality (OR, 0.78; 95%CI, 0.65-0.94) and fewer prolonged stays (OR, 0.84; 95%CI, 0.74-0.94); hospitals with an SSU had more 30-day postdischarge hospitalizations (OR, 1.19; 95%CI, 1.02-1.38); hospitals with HH had fewer prolonged stays (OR, 0.81; 95%CI, 0.73-0.89); hospitals with an HFU had more prolonged stays (OR, 1.14; 95%CI, 1.05-1.24) but fewer 30-day postdischarge ED reconsultations, hospitalizations, and combined events (OR, 0.83; 95%CI, 0.76-0.91; OR, 0.85; 95%CI=0.75-0.97, and OR, 0.84, 95%CI, 0.77-0.92); hospitals with a high-inflow ED had fewer prolonged stays (OR, 0.72; 95%CI, 0.63-0.82) as well as fewer 30-day postdischarge deaths (OR, 0.73; 95%CI, 0.56-0.96) and combined events (OR, 0.87; 95%CI, 0.77-0.92); and hospitals with an ED observation unit had more prolonged stays (OR, 1.45; 95%CI, 1.27-1.66) (figure 2).
Nonadjusted analysis of outcomes for acute heart failure patients treated in the emergency department according to hospital type
| Factor present, n (%) | Factor absent, n (%) | Odds ratio (95%CI) | P | |
|---|---|---|---|---|
| All-cause in-hospital mortality | ||||
| High-technology hospital | 1032 (7.2) | 288 (8.2) | 0.86 (0.75-0.99) | .003 |
| Hospital with a short-stay unit | 843 (7.6) | 477 (7.1) | 1.08 (0.96-1.21) | .200 |
| Hospital with home hospitalization | 951 (7.3) | 368 (7.7) | 0.93 (0.82-1.06) | .273 |
| Hospital with a heart failure unit | 727 (7.4) | 593 (7.4) | 1.01 (0.90-1.12) | .936 |
| Hospital with a high-inflow ED | 1116 (7.6) | 204 (6.4) | 1.20 (1.03-1.40) | .022 |
| Hospital with an ED observation unit | 1162 (7.5) | 158 (6.8) | 1.11 (0.93-1.31) | .251 |
| Prolonged stay (> 10 days) | ||||
| High-technology hospital | 2948 (21.2) | 944 (27.2) | 0.72 (0.66-0.79) | <.001 |
| Hospital with a short-stay unit | 2440 (22.3) | 1452 (22.6) | 0.98 (0.91-1.05) | .571 |
| Hospital with home hospitalization | 2717 (21.5) | 1175 (24.9) | 0.82 (0.76-0.89) | <.001 |
| Hospital with a heart failure unit | 2162 (22.5) | 1730 (22.3) | 1.01 (0.94-1.09) | .726 |
| Hospital with a high-inflow ED | 3107 (21.6) | 785 (26.3) | 0.77 (0.70-0.84) | <.001 |
| Hospital with an ED observation unit | 3500 (23.1) | 392 (17.6) | 1.41 (1.25-1.58) | <.001 |
| 30-day all-cause mortality | ||||
| High-technology hospital | 1372 (10.3) | 350 (10.2) | 1.01 (0.89-1.14) | .873 |
| Hospital with a short-stay unit | 1110 (10.5) | 612 (9.8) | 1.08 (0.97-1.20) | .151 |
| Hospital with home hospitalization | 1268 (10.5) | 454 (9.8) | 1.08 (0.96-1.21) | .184 |
| Hospital with a heart failure unit | 951 (10.1) | 771 (10.5) | 0.96 (0.87-1.06) | .442 |
| Hospital with a high-inflow ED | 1455 (10.5) | 267 (9.4) | 1.13 (0.99-1.30) | .080 |
| Hospital with an ED observation unit | 1514 (10.4) | 208 (9.6) | 1.09 (0.93-1.27) | .277 |
| 30-day postdischarge ED reconsultation for AHF | ||||
| High-technology hospital | 3048 (29.6) | 776 (29.0) | 1.03 (0.94-1.13) | .547 |
| Hospital with a short-stay unit | 2377 (28.5) | 1447 (31.2) | 0.89 (0.81-0.95) | .001 |
| Hospital with home hospitalization | 2672 (29.0) | 1152 (30.7) | 0.92 (0.85-1.00) | .051 |
| Hospital with a heart failure unit | 2110 (27.8) | 1714 (31.8) | 0.83 (0.76-0.89) | <.001 |
| Hospital with a high-inflow ED | 3183 (29.2) | 641 (30.7) | 0.93 (0.84-1.03) | .166 |
| Hospital with an ED observation unit | 3336 (29.7) | 488 (28.0) | 1.09 (0.97-1.22) | .145 |
| 30-day postdischarge hospitalization for AHF | ||||
| High-technology hospital | 1384 (20.6) | 431 (21.1) | 0.97 (0.86-1.09) | .603 |
| Hospital with a short-stay unit | 1208 (20.8) | 607 (20.5) | 1.02 (0.92-1.14) | .697 |
| Hospital with home hospitalization | 1262 (20.0) | 553 (22.6) | 0.86 (0.77-0.96) | .007 |
| Hospital with a heart failure unit | 1065 (20.0) | 750 (21.8) | 0.90 (0.81-1.00) | .045 |
| Hospital with a high-inflow ED | 1505 (20.7) | 310 (20.8) | 0.99 (0.87-1.14) | .913 |
| Hospital with an ED observation unit | 1618 (21.0) | 197 (18.8) | 1.15 (0.97-1.35) | .104 |
| 30-day postdischarge all-cause mortality | ||||
| High-technology hospital | 613 (5.2) | 139 (4.5) | 1.14 (0.95-1.38) | .163 |
| Hospital with a short-stay unit | 484 (5.1) | 268 (4.9) | 1.01 (0.89-1.20) | .693 |
| Hospital with home hospitalization | 568 (5.3) | 184 (4.4) | 1.21 (1.02-1.43) | .031 |
| Hospital with a heart failure unit | 422 (5.0) | 330 (5.1) | 0.98 (0.82-1.14) | .806 |
| Hospital with a high-inflow ED | 619 (5.0) | 133 (5.3) | 0.94 (0.77-1.13) | .493 |
| Hospital with an ED observation unit | 669 (5.2) | 83 (4.2) | 1.25 (0.99-1.57) | .064 |
| 30-day postdischarge combined event (death, ED reconsultation, or hospitalization) | ||||
| High-technology hospital | 3238 (31.3) | 823 (30.7) | 1.03 (0.94-1.13) | .506 |
| Hospital with a short-stay unit | 2528 (30.2) | 1533 (32.9) | 0.88 (0.82-0.95) | .001 |
| Hospital with home hospitalization | 2855 (30.8) | 1206 (32.1) | 0.94 (0.87-1.03) | .169 |
| Hospital with a heart failure unit | 2257 (29.6) | 1804 (33.4) | 0.84 (0.78-0.90) | <.001 |
| Hospital with a high-inflow ED | 3376 (30.9) | 685 (32.7) | 0.92 (0.83-1.02) | .095 |
| Hospital with an ED observation unit | 3559 (31.6) | 502 (28.8) | 1.14 (1.02-1.28) | .019 |
95%CI, 95% confidence interval; AHF, acute heart failure; ED, emergency department.
Statistical significance assigned at P<.05.
Adjusted odds ratios for the investigated outcomes according to hospital characteristics. The adjusted model included the inclusion period, patient characteristics, and hospital characteristics. Statistically significant results (P<.05) are highlighted in bold. 95%CI, 95% confidence interval; ED, emergency department.
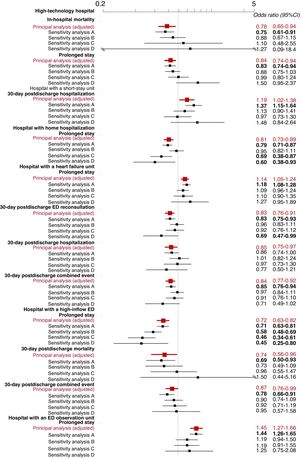
In most cases, the sensitivity analyses generated estimates showing the same trends (increased or decreased risk) as the principal analysis. However, these analyses included fewer patients (17 974 in the principal analysis vs 13 347, 8615, 3828, and 3427 in sensitivity analysis A, B, C, and D, respectively), and therefore the 95%CIs were wider and many included the value 1 (figure 3). In contrast, LVEF mostly showed no interactions with the statistically significant associations in the principal analysis; the exceptions were the association between high-technology hospitals and fewer prolonged stays, which was more evident in patients with LVEF ≥ 50% (OR, 0.89; 95%CI, 0.55-1.44) than in those with LVEF<50% (OR, 1.43; 95%CI, 0.96-2.13; interaction, P=.032), and the association between hospitals with an SSU and more 30-day postdischarge hospitalizations, which was more evident in patients with LVEF<50% (OR, 1.39; 95%CI, 0.89-2.17) than in those with LVEF ≥ 50% (OR, 0.86; 95%CI, 0.50-1.45; interaction, P=.035).
Sensitivity analysis of associations between hospital characteristics and acute heart failure patient outcomes that were statistically significant in the adjusted principal analysis. Statistically significant results (P<.05) are highlighted in bold. Sensitivity analysis A exclusively included the 13 347 patients admitted to hospital for AHF (excluding patients discharged directly from the ED). Sensitivity analysis B exclusively included the 8615 patients whose AHF was confirmed by natriuretic-peptide assay. Sensitivity analysis C limited multiple imputation to variables with <10% of missing patient variables, corresponding to a total of 3828 patients. Sensitivity analysis D was performed without multitple imputation of missing values, limiting the analysis to 3427 patients. 95%CI, 95% confidence interval; ED, emergency department.
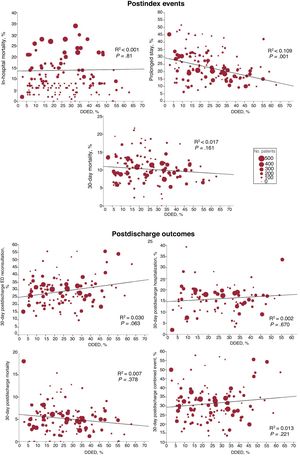
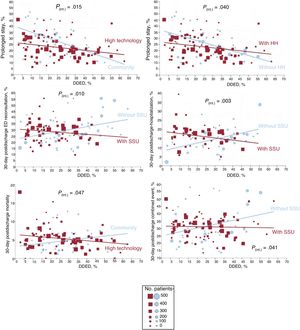
The higher the percentage of DDED, the fewer the prolonged stays (R2=0.109; P<.001), but the DDED rate showed no significant association with the other outcomes (figure 4). In the stratified analysis according to hospital and ED structural/organizational characteristics, DDED showed significant interaction with 6 of the 42 relationships analyzed (), grouped as follows: a) hospital type for prolonged stay (a more intense decrease in community hospitals, P=.015) and for 30-day postdischarge mortality (decreasing in high-technology hospitals and increasing in community hospitals, P=.047); b) the presence of an HH service for prolonged stay (a more intense decrease if there was no HH, P=.04); and c) the presence of an SSU for 30-day postdischarge ED reconsultations, hospitalizations, and combined events (declining if there was an SSU and increasing if there was not; P=.01, P=.003, and P=.041, respectively) (figure 5).
The present study shows that hospitals and ED structural and organizational characteristics significantly impact the short-term outcomes of patients diagnosed with AHF in the ED, irrespective of the inclusion period or the profile of patients attending each ED. Indeed, adjustment for these factors did not substantially alter the nonadjusted calculations. This may indicate a similar distribution of AHF patients between inclusion periods and participating centers, which would be consistent with the high prevalence of AHF and the fact that most patients are treated at their nearest hospital, with few patients transferred to a higher level of care. It may also be that between-center differences in the distribution of some patient characteristics had opposing effects on outcomes that largely canceled each other out. Taken together, the main findings discussed below provide a useful framework for planning selected organizational features of AHF patient management.
The first notable finding is that prolonged stays related to the index event were less frequent at high-technology hospitals and hospitals with an HH service or a high-inflow ED. The good performance of high-technology hospitals may reflect an ability to carry out certain examinations more rapidly, especially if the patient is admitted; however, this possibility was not investigated in the present study. Probably the most important factor limiting prolonged stays after the index event is the high caseload and greater experience of high-inflow EDs, which results in more decisions to discharge directly from the ED. These results also confirm the effectiveness of structures specifically aimed at limiting length of stay, such as HH, at least in the case of AHF. On the other hand, the number of prolonged stays was increased by the presence of an HFU or an ED observation unit. The presence of these facilities might suggest more conservative management of AHF patients, whereas hospitals lacking an HFU tend to favor more symptom-focused care and to discharge patients without completing diagnostic and therapeutic procedures. Our study was not designed to explore these questions, and they should therefore be tested in specifically designed studies in the future.
Uniquely among hospital and ED structural/organizational characteristics, high-technology was associated with significantly lower in-hospital mortality. However, this advantage of high-technology hospitals was not seen for 30-day mortality or 30-day postdischarge mortality, suggesting that the lower in-hospital mortality may reflect differential management of patients showing good progress versus those who are dependent or in a terminal condition. Differential patient management of this type is common practice with AHF patients,14,15 and high-technology hospitals may as a consequence have more structured extrahospital care programs that increase the proportion deaths occurring after discharge.
The presence of an HFU shows a clear relationship with better postdischarge outcomes. While this important finding has been described in previous reports,7,16 the present results demonstrate that the association is independent of other characteristics that may be present in the same hospital and could also influence prognosis. In light of our results, we believe that HFUs should be established more extensively within the Spanish health system because, beyond their benefits for patients with complex forms of HF during the stabilization period, these specialist units provide a clear benefit to patients with acute decompensated HF.
A high DDED rate correlated with fewer prolonged stays. Although a more aggressive patient management might be expected to result in worse postdischarge outcomes, this expectation is not supported by the 30-day postdischarge data. Some hospital or ED structural/organizational features may even have minimized this potential negative effect. For example, our data indicate that the presence of an SSU allows for safer discharge from the ED, which may also be a benefit of high-technology hospitals. AHF patients are currently not risk-stratified before the decision on discharge or hospital admission from the ED; our findings suggest that systematic risk stratification would contribute to improved selection of patients for discharge while maintaining the rate of DDED.11,17–19
LimitationsThis was a retrospective analysis, and the results should therefore be considered hypothesis generating. A second limitation is the possibility of bias in the selection of participating centers, since EDs joined the EAHFE Registry on a voluntary basis. Third, AHF diagnosis in the EAHFE Registry was essentially based on clinical data and was not always confirmed by analytical data or echocardiography, partially limiting internal validity. Given the results of the TOPCAT study,20 the failure to confirm diagnosis by natriuretic peptides in 52% of patients is especially significant. Nevertheless, sensitivity analysis B included only patients with a natriuretic–peptide-confirmed diagnosis and yet generated results similar to those of the principal analysis, suggesting that the lack of diagnostic confirmation likely had a limited impact on the results. A fourth limitation is related to the inclusion of patients in the ED and the associated predominance of patients in whom LVEF (known in only 52% of patients) was preserved; this profile differs from that of patients admitted for AHF, especially to cardiology units, and therefore raises the question of external validity. Moreover, the subgroup analysis shows a different behavior for some outcome measures in patients with preserved LVEF. A fifth limitation is that, with the exception of placement of a cardiac resynchronization pacemaker, no data were collected on specific treatments or complementary tests, including those targeting decompensation triggers (such as acute coronary syndrome), which may be treated earlier or more aggressively in high-technology hospitals. Finally, some calculations may have been affected by type I errors as a result of the performance of multiple comparisons with no adjustment to prioritize the exploratory nature of the study.
CONCLUSIONSThe short-term outcomes of AHF patients are strongly affected by hospital and ED structural and organizational characteristics and by the aggressiveness of ED management, indexed by the percentage of DDED. Although the present study did not include all examinations and treatments during hospitalization, we believe that health care managers should use these findings to strengthen measures to improve AHF patient prognosis. Among these measures, the presence of an HFU is of particular interest.
CONFLICTS OF INTERESTNone.
- -
There is well-established knowledge about how AHF prognosis is affected by factors related to patient baseline status and the decompensation episode.
- -
Both generally and in Spain, previous reports have not examined the combined effects of factors related to health care organization, especially those related to the hospital or ED first treating the patient.
- -
After an index AHF episode (initially treated in the ED), prolonged stays are less frequent at high-technology hospitals and hospitals with an SSU, an HH service, or a high-inflow ED.
- -
High-technology hospitals have lower in-hospital mortality; high-inflow EDs have lower 30-day postdischarge mortality; and hospitals with an HFU have lower 30-day postdischarge rates of ED reconsultation, hospitalization, and combined events.
- -
A higher the rate of DDED is associated with fewer prolonged stays but more postdischarge reconsultations, although this relationship is not seen at hospitals with an SSU.
This study was made possible in part due to support from the Instituto de Salud Carlos III through Spanish Ministry of Health and ERDF funding (PI15/01019, PI18/00773), La Marató de TV3 (2015/2510), and the Autonomous Government of Catalonia program for consolidated research groups (GRC 2009/1385, 2014/0313, 2017/1424).
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.11.022