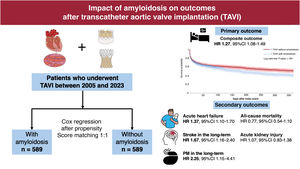
Data on the impact of amyloidosis on outcomes after transcatheter aortic valve implantation (TAVI) are limited. The objective of this study was to evaluate the 1-year risk of adverse events post-TAVI in patients with amyloidosis.
MethodsPatients undergoing TAVI (between 2005 and 2023) were categorized into 2 groups based on the presence of amyloidosis. The primary outcome was the 1-year risk of a composite endpoint: heart failure (HF), ischemic stroke, pacemaker implantation, acute kidney injury, and all-cause death. Secondary outcomes assessed the individual components of the composite. Propensity score matching was used to balance the groups, and Cox regression was used to assess the risk of adverse outcomes associated with amyloidosis. Composite outcomes were analyzed for early (30-days) and long-term (30-days to 1 year) follow-up.
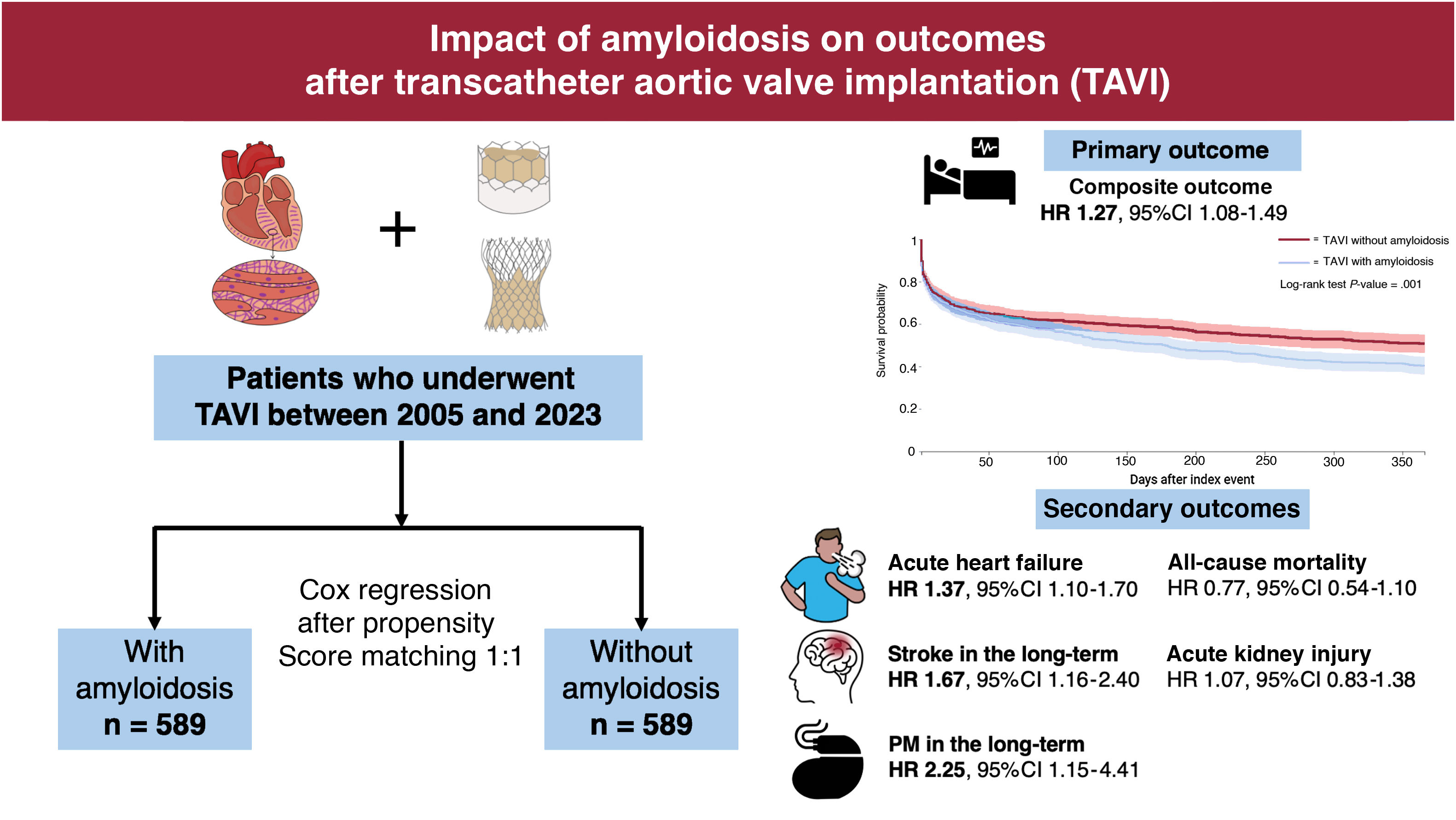
ResultsData from 589 TAVI patients with amyloidosis (mean age 78.9±8.2 years, 31.9% female) were compared with 52 296 individuals without amyloidosis (mean age 78.1±8.8 years, 40.3% female). After propensity score matching, patients with amyloidosis had a significantly higher 1-year risk of adverse events (HR, 1.27; 95%CI, 1.08-1.49). Specifically, patients with amyloidosis showed an increased risk of HF (HR, 1.37; 95%CI, 1.10-1.70). Stroke risk (HR, 1.67; 95%CI, 1.16–2.40) and pacemaker implantation (HR, 2.25; 95%CI, 1.15-4.41) were higher during long-term follow-up, while no differences were found for acute kidney injury or all-cause mortality between the 2 groups.
ConclusionsAmong patients undergoing TAVI, those with amyloidosis are at a higher risk of adverse events, particularly HF, and have an increased risk of pacemaker implantation and stroke in the long-term.
Keywords
Amyloidosis is a progressive, systemic disease caused by misfolded proteins that progressively accumulate in the interstitial space as insoluble β-sheet amyloid fibrils.1 Cardiac amyloidosis (CA) is caused by the deposition of fibrils in the myocardium, the conduction system of the heart, and the valvular tissue.2 A significant proportion of patients with aortic stenosis (approximately 16%)— especially those undergoing transcatheter aortic valve implantation (TAVI)—have been found to have transthyretin associated (ATTR) cardiomyopathy3,4 and, with a lower prevalence, light chain CA.5
However, the effect of amyloid fibrils on structural valve deterioration and on the clinical outcomes of patients who undergo TAVI is still largely unclear. The few studies on this topic have yielded conflicting results.6–8 For example, Scully et al.6 reported no differences in the periprocedural complications of TAVI between patients with and without amyloidosis, whereas a recent meta-analysis showed that amyloidosis was associated with an increased risk of all-cause mortality and other common complications, such as stroke, heart failure (HF), and acute kidney injury (AKI).7 Given these discrepancies, further studies are needed to elucidate these questions.
We therefore aimed to investigate the 1-year risk of adverse events following TAVI in patients with amyloidosis using a large global federated health research network.
METHODSTriNetX is a global network of health care organizations compliant with the HIPAA (Health Insurance Portability and Accountability Act) and the United States federal law which protects the privacy and security of health care data. All data are deidentified in accordance with the deidentification standards outlined in the HIPAA Privacy Rule. A data-sharing agreement is needed to access the data in the TriNetX network. As a federated research network with non-identifiable patient data, studies conducted using the TriNetX health research network do not require approval from an ethics committee. TriNetX does not modify, impute, or estimate missing clinical values to address gaps in patient records. Additional details regarding data extraction from TriNetX are provided in the .
Study designWe conducted a retrospective observational study within TriNetX, using electronic medical records from academic medical centers, specialty physician practices, and community hospitals, encompassing data from more than 180 million individuals, mainly in the United States. The available data from this global network include demographics, diagnoses coded with the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), procedures coded using Current Procedural Terminology, and medications recorded using Veteran Affairs codes.
CohortQueries on the TriNetX network's online platform were conducted on September 10, 2024, targeting patients aged ≥18 years who underwent TAVI (Current Procedural Terminology code 1021150). Using ICD-10-CM codes, we created 2 cohorts: a) TAVI recipients with amyloidosis (E85) and b) TAVI recipients without amyloidosis ().
The study period spanned from January 1, 2005, to December 31, 2023. On the date of the search, data from 88 healthcare organizations were available for patients meeting the inclusion and exclusion criteria. The index event, defined as the time point when each patient entered the analysis, was the date of their TAVI. Any diagnoses or treatments recorded prior to the index event were considered part of the individual's baseline characteristics.
OutcomesThe primary outcome was the 1-year risk of a composite endpoint consisting of all-cause death, or acute HF, or ischemic stroke, or pacemaker (PM) implantation, or AKI. The secondary outcomes were the 1-year risks of each individual component of the primary outcomes. Further details about the ICD-10-CM codes used to identify these adverse events are provided in .
Statistical analysisAll statistical analyses were performed using the TriNetX online research platform. Baseline characteristics of patients with and without amyloidosis were balanced through propensity score matching (PSM) using logistic regression, with a 1:1 ratio. The greedy nearest neighbor method with a caliper of 0.1 pooled standard deviations was applied. The following variables were included in the PSM: age, sex, ethnicity, hypertension, dyslipidemia, diabetes mellitus, HF, atrial fibrillation (AF) and atrial flutter, ischemic heart disease, prior cardiac surgery, concomitant valvular diseases, baseline conduction disturbances, cerebral infarction, pulmonary embolism, peripheral artery disease, obesity, chronic kidney disease, and cardiovascular medications (including beta-blockers, digitalis, calcium-channel blockers, diuretics, antilipemic agents, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, anticoagulants, and antiplatelets).
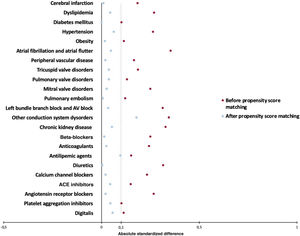
Absolute standardized mean differences (ASD) were used to assess the distribution of demographic and clinical characteristics between the groups. ASD was calculated as the difference in means or proportions of a given variable divided by the pooled estimate of the standard deviation (SD) for that variable. A baseline characteristic with an ASD <0.1 was considered well matched. A plot of the propensity score distributions before and after matching was included to show the degree of overlap between the groups.
After PSM, univariable Cox proportional hazards analysis was performed to calculate hazard ratios (HRs) and 95% confidence intervals (95% CI) for the risk of adverse events in TAVI patients with amyloidosis compared with those without amyloidosis. Kaplan-Meier survival curves and the Log-Rank test were also generated to illustrate differences in event-free survival distributions between patients with and without amyloidosis and to assess the significance of these differences, respectively.
To assess whether the proportional hazards assumption held in the Cox regression model, we applied a chi-square test based on Schoenfeld residuals. Details on how the test was performed are provided in the . When the proportional hazards assumption for the primary outcome was not satisfied, we divided the 1-year follow-up period into 2 phases: an early phase (the first 30 days of follow-up) and a late phase (from the end of the first month to the end of the first year). We then re-evaluated the risk using Cox regression and retested the proportional hazards assumption for each phase.
To assess the unadjusted cumulative incidence of each secondary outcome and to evaluate the relationship between nonfatal and fatal events, we performed Aalen-Johansen estimator curves.
To account for advances in TAVI techniques over time and their impact on the risk of adverse events in patients with amyloidosis, we investigated the risk of primary and secondary outcomes in 2 distinct time periods: early (2005-2019) and late (2020-2023).
All tests were 2-tailed and P-values <.05 were considered statistically significant. All analyses were performed in the TriNetX platform, which uses R's survival package v3.2-3.
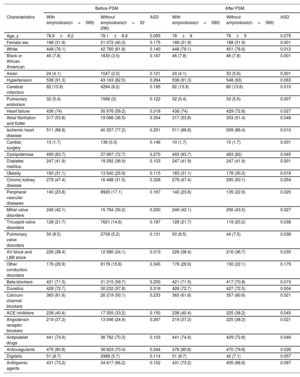
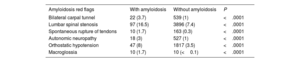
RESULTSOur study included 589 TAVI patients with amyloidosis (mean age±SD 78.9±8.2 years, 31.9% female) and 52 296 TAVI patients without amyloidosis (mean age±SD 78.1±8.8 years, 40.3% female). Before PSM, patients with amyloidosis were more likely to be male, Black, or African American, or Asian and had a significantly higher prevalence of cardiovascular risk factors, HF, AF, conduction system diseases, previous cerebral infarction, pulmonary embolism, and chronic kidney disease, compared with those without amyloidosis (table 1). These patients had a higher prevalence of bilateral carpal tunnel syndrome (3.7% vs 1%, P<.0001), lumbar spinal stenosis (16.5% vs 7.4%, P<.0001), spontaneous rupture of tendons (1.7% vs 0.3%, P<.0001), orthostatic hypotension (8% vs 3.5%, P<.0001), and macroglossia (1.7% vs 0.01%, P<.0001), which are considered the most common clinical red flags for CA9 (table 2).
Baseline characteristics of TAVI patients with (N=589) and without (N=52 296) amyloidosis before and after propensity score matching
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Characteristics | With amyloidosis(n=589) | Without amyloidosis(n=52 296) | ASD | With amyloidosis(n=589) | Without amyloidosis(n=589) | ASD |
| Age, y | 78.9±8.2 | 78.1±8.8 | 0.095 | 78±8 | 78±9 | 0.079 |
| Female sex | 188 (31.9) | 21 072 (40.3) | 0.175 | 188 (31.9) | 188 (31.9) | 0.001 |
| White | 448 (76.1) | 42 760 (81.8) | 0.140 | 448 (76.1) | 451 (76.6) | 0.012 |
| Black or African American | 46 (7.8) | 1830 (3.5) | 0.187 | 46 (7.8) | 46 (7.8) | 0.001 |
| Asian | 24 (4.1) | 1047 (2.0) | 0.121 | 24 (4.1) | 33 (5.6) | 0.001 |
| Hypertension | 538 (91.3) | 43 163 (82.5) | 0.264 | 538 (91.3) | 548 (93) | 0.063 |
| Cerebral infarction | 82 (13.9) | 4264 (8.2) | 0.185 | 82 (13.9) | 80 (13.6) | 0.010 |
| Pulmonary embolism | 32 (5.4) | 1566 (3) | 0.122 | 32 (5.4) | 33 (5.6) | 0.007 |
| Heart failure | 436 (74) | 30 976 (59.2) | 0.318 | 436 (74) | 429 (72.8) | 0.027 |
| Atrial fibrillation and flutter | 317 (53.8) | 19 086 (36.5) | 0.354 | 317 (53.8) | 303 (51.4) | 0.048 |
| Ischemic heart disease | 511 (86.8) | 40 357 (77.2) | 0.251 | 511 (86.8) | 509 (86.4) | 0.010 |
| Cardiac surgery | 10 (1.7) | 136 (0.3) | 0.146 | 10 (1.7) | 10 (1.7) | 0.001 |
| Dyslipidemias | 493 (83.7) | 37 997 (72.7) | 0.270 | 493 (83.7) | 483 (82) | 0.045 |
| Diabetes mellitus | 247 (41.9) | 19 292 (36.9) | 0.103 | 247 (41.9) | 247 (41.9) | 0.001 |
| Obesity | 183 (31.1) | 13 540 (25.9) | 0.115 | 183 (31.1) | 178 (30.2) | 0.018 |
| Chronic kidney disease | 279 (47.4) | 16 488 (31.5) | 0.328 | 279 (47.4) | 295 (50.1) | 0.054 |
| Peripheral vascular diseases | 140 (23.8) | 8920 (17.1) | 0.167 | 140 (23.8) | 135 (22.9) | 0.020 |
| Mitral valve disorders | 248 (42.1) | 15 784 (30.2) | 0.250 | 248 (42.1) | 256 (43.5) | 0.027 |
| Tricuspid valve disorders | 128 (21.7) | 7621 (14.6) | 0.187 | 128 (21.7) | 119 (20.2) | 0.038 |
| Pulmonary valve disorders | 50 (8.5) | 2709 (5.2) | 0.131 | 50 (8.5) | 44 (7.5) | 0.038 |
| AV block and LBB block | 226 (38.4) | 12 580 (24.1) | 0.313 | 226 (38.4) | 216 (36.7) | 0.035 |
| Other conduction disorders | 176 (29.9) | 8178 (15.6) | 0.345 | 176 (29.9) | 130 (22.1) | 0.179 |
| Beta-blockers | 421 (71.5) | 31 215 (59.7) | 0.250 | 421 (71.5) | 417 (70.8) | 0.015 |
| Diuretics | 428 (72.7) | 30 232 (57.8) | 0.316 | 428 (72.7) | 427 (72.5) | 0.004 |
| Calcium-channel blockers | 363 (61.6) | 26 219 (50.1) | 0.233 | 363 (61.6) | 357 (60.6) | 0.021 |
| ACE inhibitors | 238 (40.4) | 17 355 (33.2) | 0.150 | 238 (40.4) | 225 (38.2) | 0.045 |
| Angiotensin receptor blockers | 219 (37.2) | 13 046 (24.9) | 0.267 | 219 (37.2) | 225 (38.2) | 0.021 |
| Antiplatelet drugs | 441 (74.9) | 36 762 (70.3) | 0.103 | 441 (74.9) | 429 (72.8) | 0.046 |
| Anticoagulants | 476 (80.8) | 36 823 (70.4) | 0.244 | 476 (80.8) | 470 (79.8) | 0.026 |
| Digitalis | 51 (8.7) | 2988 (5.7) | 0.114 | 51 (8.7) | 42 (7.1) | 0.057 |
| Antilipemic agents | 431 (73.2) | 34 617 (66.2) | 0.152 | 431 (73.2) | 405 (68.8) | 0.097 |
AV, atrioventricular; ACE, angiotensin-converting enzyme inhibitors; ASD, absolute standardized mean difference; CABG, coronary artery bypass graft, LBB, left bundle branch, PSM, propensity score matching.
Data are expressed as No. (%) or mean±standard deviation.
Clinical red flags for amyloidosis in the 2 groups identified for the analysis
| Amyloidosis red flags | With amyloidosis | Without amyloidosis | P |
|---|---|---|---|
| Bilateral carpal tunnel | 22 (3.7) | 539 (1) | <.0001 |
| Lumbar spinal stenosis | 97 (16.5) | 3896 (7.4) | <.0001 |
| Spontaneous rupture of tendons | 10 (1.7) | 163 (0.3) | <.0001 |
| Autonomic neuropathy | 18 (3) | 527 (1) | <.0001 |
| Orthostatic hypotension | 47 (8) | 1817 (3.5) | <.0001 |
| Macroglossia | 10 (1.7) | 10 (<0.1) | <.0001 |
Data are expressed as No. (%).
After PSM, with 589 patients in each cohort, no significant differences were observed between the 2 groups in terms of demographics, prescribed medications, and diagnoses, except for the prevalence of conduction system diseases, which remained higher in patients with amyloidosis compared with those without (29.9% vs 22.1%, respectively; ASD: 0.179) (table 1). Figure 1 provides a visual assessment of how PSM reduced differences between the 2 groups, ensuring that the effects observed were less likely to be due to confounding factors.
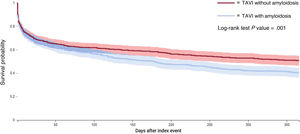
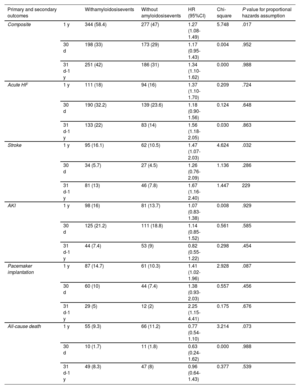
Table 3 shows the number of events following TAVI in patients with and without amyloidosis and the HRs, with relative 95%CI, for the primary and secondary outcomes at the 1-year follow-up. As shown by the Kaplan-Meier curves, patients diagnosed with amyloidosis had a significantly higher 1-year risk of the composite outcome compared with controls (HR, 1.27; 95%CI, 1.08-1.49) (figure 2). However, when analyzing the proportional hazards assumption, we observed substantial variability in the risk estimates compared with those expected (chi-square 5.75; P prop.=.017). This violation of the proportional hazards assumption suggests that the impact of amyloidosis on outcomes after TAVI may vary over time. When dividing the follow-up into 2 periods, we found that the risk of the composite outcome in patients with amyloidosis was greater during the late phase of follow-up (HR, 1.34; 95%CI, 1.11-1.61) compared with the early phase (HR, 1.17; 95%CI, 0.95-1.43), with no violation of the proportional hazards assumption in either period (table 3).
Risk of primary and secondary outcomes in patients with and without amyloidosis after propensity score matching in the first year, in the first month and between 31 days and 1 year of follow-up after transcatheter aortic valve implantation
| Primary and secondary outcomes | Withamyloidosisevents | Without amyloidosisevents | HR (95%CI) | Chi-square | P value for proportional hazards assumption | |
|---|---|---|---|---|---|---|
| Composite | 1 y | 344 (58.4) | 277 (47) | 1.27 (1.08-1.49) | 5.748 | .017 |
| 30 d | 198 (33) | 173 (29) | 1.17 (0.95-1.43) | 0.004 | .952 | |
| 31 d-1 y | 251 (42) | 186 (31) | 1.34 (1.10-1.62) | 0.000 | .988 | |
| Acute HF | 1 y | 111 (18) | 94 (16) | 1.37 (1.10-1.70) | 0.209 | .724 |
| 30 d | 190 (32.2) | 139 (23.6) | 1.18 (0.90-1.56) | 0.124 | .648 | |
| 31 d-1 y | 133 (22) | 83 (14) | 1.56 (1.18-2.05) | 0.030 | .863 | |
| Stroke | 1 y | 95 (16.1) | 62 (10.5) | 1.47 (1.07-2.03) | 4.624 | .032 |
| 30 d | 34 (5.7) | 27 (4.5) | 1.26 (0.76-2.09) | 1.136 | .286 | |
| 31 d-1 y | 81 (13) | 46 (7.8) | 1.67 (1.16-2.40) | 1.447 | 229 | |
| AKI | 1 y | 98 (16) | 81 (13.7) | 1.07 (0.83-1.38) | 0.008 | .929 |
| 30 d | 125 (21.2) | 111 (18.8) | 1.14 (0.85-1.52) | 0.561 | .585 | |
| 31 d-1 y | 44 (7.4) | 53 (9) | 0.82 (0.55-1.22) | 0.298 | .454 | |
| Pacemaker implantation | 1 y | 87 (14.7) | 61 (10.3) | 1.41 (1.02-1.96) | 2.928 | .087 |
| 30 d | 60 (10) | 44 (7.4) | 1.38 (0.93-2.03) | 0.557 | .456 | |
| 31 d-1 y | 29 (5) | 12 (2) | 2.25 (1.15-4.41) | 0.175 | .676 | |
| All-cause death | 1 y | 55 (9.3) | 66 (11.2) | 0.77 (0.54-1.10) | 3.214 | .073 |
| 30 d | 10 (1.7) | 11 (1.8) | 0.63 (0.24-1.62) | 0.000 | .988 | |
| 31 d-1 y | 49 (8.3) | 47 (8) | 0.96 (0.64-1.43) | 0.377 | .539 |
95%CI, 95% confidence interval; AKI, acute kidney injury; HF, heart failure; HR, hazard ratio.
Data are expressed as No. (%).
When considering the secondary outcomes, patients with amyloidosis were more likely to experience acute HF episodes (HR, 1.37; 95%CI, 1.10-1.70, chi-square 0.124 P prop.=.724) throughout the follow-up period, with no violation of the proportional hazards assumption.
The risk of PM implantation showed an increasing trend during long-term follow-up. Specifically, patients with amyloidosis had a higher overall risk of PM implantation (HR, 1.41; 95%CI, 1.02-1.95), but with a proportional hazards assumption at the threshold of significance (chi-square=2.92, P prop.=.087). When considering only the late follow-up period (31 days to 1 year), the risk of PM implantation tended to rise, with no violation of proportionality (HR, 2.25; 95%CI, 1.15-4.41, chi-square=0.175, P prop.=.676).
Patients with amyloidosis also demonstrated an increased risk of stroke following TAVI. Specifically, the risk was higher for the entire observation period (HR, 1.47; 95%CI, 1.07-2.03, chi-square=4.624 P prop.=.032) with a slight violation of proportionality. However, when considering the long-term follow-up period, the risk of stroke remained consistently higher with proportionality respected (HR, 1.67; 95%CI, 1.16–2.40, chi-square=1.447 P prop.=.229).
No statistically significant differences were observed between the 2 groups regarding the risks of AKI (HR, 1.07; 95%CI, 0.83–1.38), with no violation of proportionality (chi square=0.008; P prop=0929). Similarly, no significant difference was found for the risk of all-cause mortality (HR, 0.77; 95%CI, 0.54–1.10), although this outcome showed high variability over the observation period (chi-square=3.21; P prop.=.073) (figure 3).
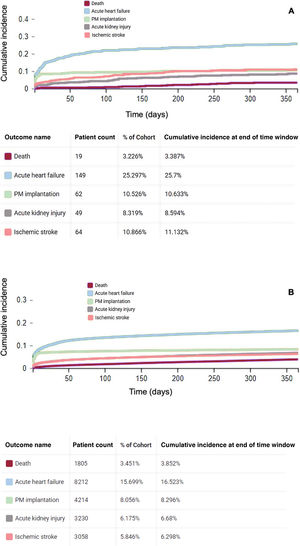
As shown by the Aalen-Johansen curves, the cumulative incidence at the end of follow-up for the different outcomes in patients with amyloidosis was 3.4% for all-cause death, 25.7% for HF, 10.6% for PM implantation, 8.6% for AKI, and 11.1% for ischemic stroke. In comparison, the cumulative incidence for patients without amyloidosis was 3.8% for all-cause death, 16.5% for HF, 8.3% for PM implantation, 6.7% for AKI, and 6.3% for ischemic stroke (figure 4).
Considering the advances in TAVI techniques over time, we divided the cohort into 2 periods: an early period (2005-2019) and a later period (2020-2023). This approach confirmed that the main analysis results were not significantly affected by recent improvements in TAVI. As shown in , findings from both periods were consistent with the overall results (composite HR, 1.27; 95%CI, 1.08-1.49). Specifically, the HR for 2005-2019 was 1.35 (95%CI, 1.02-1.78, chi- square=0.86, P prop.=.353), while for 2020-2023, it was 1.17 (95%CI, 1.00-1.42, chi-square=0.31; P prop.=.579), indicating similar trends in both periods, without significant violation of the proportional hazards assumption.
DISCUSSIONIn the present observational study, we provide clinical insights into a patient population undergoing TAVI with a diagnosis of amyloidosis. Our main findings are as follows: a) amyloidosis was associated with an increased risk of adverse events within the first year post-TAVI; b) the risk was not constant over time and appeared to increase progressively during the later phase of the follow-up period; c) the risk of HF remained steady throughout the follow-up period; d) over the long-term, amyloidosis was associated with a higher risk of PM implantation and stroke (figure 5).
Central illustration. Amyloid deposition in the heart might increase the risk of adverse events in the first year following TAVI. Patients with amyloidosis are at higher risk of heart failure, stroke, and pacemaker implantation. 95%CI, 95% confidence interval; HR, hazard ratio; TAVI, transcatheter aortic valve implantation.
In our population, the prevalence of amyloidosis in patients undergoing TAVI was <1%. This value is significantly lower than those reported by other studies, where the prevalence of CA in TAVI candidates was approximately 8% to 16%.3,10 This discrepancy could be explained by various factors. First, screening methods for amyloidosis detection are not uniformly applied, not only among different health care organizations, but also over time. Indeed, in the past, there was a lack of awareness that a large proportion of patients undergoing TAVI have concomitant amyloidosis; echocardiographic red flags for amyloidosis have only recently been extensively recommended11 and specific ICD-10-CM codes for ATTR-CA and AL-CA have been introduced only since 2018, leading to underreporting of the disease. Second, imaging techniques applied for screening have different sensitivities and specificities. For example, it has been reported that cardiac magnetic resonance has lower sensitivity than bone scintigraphy.12 When patients are systematically screened for ATTR-CA using bone scintigraphy before the procedure, the prevalence of CA drastically increases.6,8,13
Despite the low prevalence of amyloidosis in the analysis, our results are in line with those of previous studies supporting the hypothesis that, although TAVI improves outcomes independently of the presence of amyloidosis,14 CA in TAVI candidates is associated with a higher rate of adverse events after valve replacement.
In our study, patients with amyloidosis were more susceptible to worsening HF, experiencing a higher rate of acute episodes during both short- and long-term follow-up after the intervention. Although the lack of information about left and right ventricular ejection fraction at baseline and during follow-up may limit the generalizability of our results, this finding appears to be supported by previous reports. In a prospective multicenter observational study of 27 patients with ATTR-CA undergoing TAVI, Rosenblum et al.8 reported that, although mortality was similar to that in controls, patients with amyloidosis had an increased risk of hospitalization for HF 1 year and 3 years after TAVI. These findings may be explained by amyloid deposits in the myocardial extracellular space, which progressively stiffen the ventricles, leading to both diastolic and systolic dysfunction. This impairs the ability of the heart to reverse remodeling after valve replacement and increases the risk of postoperative HF.15
In this context, as reported by histopathological studies,16 it should be considered that amyloid fibrils frequently deposit in the valvular tissue, causing a proinflammatory state that induces the differentiation of valvular interstitial cells into myofibroblasts and osteoclast-like cells, which perpetuate fibrosis and calcification of the extracellular matrix.17 The same pathological mechanisms could be associated with the degeneration of biological valve prostheses, but these hypotheses require further investigation.18
The AMYLOCARTESIAN trial (Prevalence and Post-surgical Outcomes of Cardiac Wild-type Transthyretin Amyloidosis in Elderly Patients With Aortic Stenosis Referred for Valvular Replacement; NCT02260466) is an ongoing multicenter prospective study that might help clarify the precise effect of amyloid fibrils on the myocardium and prosthetic valves.
We found that following TAVI, patients with amyloidosis have an increased risk of stroke—not periprocedural, but in the long-term follow-up. In a retrospective observational study on 273 TAVI patients with concomitant amyloidosis, Elzeneini et al.,19 found that CA was associated with a 3-fold increased risk of acute ischemic stroke during index hospitalization (odds ratio [OR], 3.08; 95%CI, 1.41-6.71). Conversely, Ismayl et al.20 did not find a significant association between amyloidosis and stroke following TAVI. A meta-analysis that included these 2 studies21 concluded that there was a significant increase in stroke risk in patients with a diagnosis of amyloidosis undergoing TAVI compared with those with lone aortic stenosis (OR, 1.97; 95%CI, 1.05-3.69).
Several factors might explain this increased risk. First, amyloid deposition in the valvular tissue, together with valve calcifications, could increase the amount of debris released from the native valve during prosthesis implantation.17 Second, due to amyloid deposition in the atrial myocardium and subsequent atrial cardiomyopathy, CA itself carries per se an increased risk of thrombus formation in the cardiac chambers.22,23 The use of cerebral embolic protection during the TAVI procedure is still a matter of debate regarding which patient subgroups might benefit from this strategy.24 Moreover, antithrombotic treatment following TAVI could be tailored to patients’ specific comorbidities, including amyloidosis.25 To date, the antithrombotic strategy used after TAVI in patients who do not have an indication for oral anticoagulant therapy involves single antiplatelet therapy with aspirin.26 However, it is still debated whether more effective antithrombotic therapy is necessary in specific categories of patients at greater ischemic risk. Although data from randomized trials support anticoagulant therapy only in patients diagnosed with AF,27–29 early diagnosis of CA associated with severe cardiomyopathy during the pre-TAVI screening could influence antithrombotic therapy decisions to reduce the incidence of thromboembolic events after the intervention.
Another relevant finding in our study was that amyloidosis increased the risk of permanent PM implantation mainly within 31 days to 1 year after TAVI. This could mainly be due to the higher prevalence of conduction system disorders at baseline, which we observed in patients with amyloidosis compared with those without, even after PSM. However, it should be noted that complete atrioventricular blocks and new-onset left or right bundle branch block are often the result of the valve replacement itself,30 potentially further exacerbating the need for PM implantation in a significant proportion of patients with amyloidosis. Our results appear to support this hypothesis, suggesting that amyloidosis may increase the long-term risk of conduction system diseases due to amyloid fibril deposition in the conduction system, as recently demonstrated by a systematic review and meta-analysis.31 The diagnosis of amyloidosis, often associated with aortic stenosis prior to intervention, could affect valve choice. Knowing that a patient has amyloidosis might lead to the choice of balloon-expandable valves in this category of patients, as some studies suggested that self-expandable valves could be associated with a higher risk of conduction disorders.32–34 Moreover, patients at high risk of atrioventricular block, such as those with CA, might require more careful and prolonged electrocardiogram monitoring following the procedure.
The risk of AKI, a significant complication of TAVI, was not significantly higher in patients with amyloidosis in our cohort during the first year after valve replacement compared with those without. These findings differ from earlier studies that reported a higher AKI rate in this population. Further research is needed to determine whether patients with amyloidosis are more susceptible to contrast-induced nephropathy.
In addition, mortality was not significantly affected by the presence of amyloidosis, as reported in a previous observational study.6 However, this could be related to the small number of patients considered and the relatively short follow-up period after the index event, which may have limited the statistical power of our study.
Lastly, in the sensitivity analysis, which evaluated the cohorts in 2 different periods (2005-2019 and 2020-2023), we demonstrated that the results from the main analysis were consistent over time and that, while TAVI techniques have undoubtedly improved, the underlying association between amyloidosis and the outcome of interest remained stable. The slightly lower HR in the 2020 to 2023 period may reflect better procedural outcomes due to technological advances, but it does not significantly alter the overall conclusion.
Clinical implicationsThe awareness that CA might be associated with an increased risk of adverse events following TAVI should prompt cardiologists and imaging experts to look for clinical and echocardiographic red flags of the disease during the diagnostic work-up of severe aortic stenosis.14 In this population, the diagnosis of CA might be challenging30; however in the era of machine learning, artificial intelligence could facilitate systematic screening.31,32 Such an approach might influence several periprocedural and procedural choices. Nitsche et al.33 demonstrated that ATTR-CA is associated with blunted echocardiographic remodeling and reduced regression of symptoms after TAVI. Identifying this subgroup of TAVI candidates could be crucial to introduce, even before valve replacement, novel ATTR-specific (disease modifying) therapies. Tafamidis, a TTR tetramer stabilizer, has been demonstrated to reduce mortality and rehospitalization in patients with transthyretin amyloid cardiomyopathy34 and it is likely that this molecule will improve outcomes even in ATTR-CA patients undergoing TAVI.35
LimitationsSeveral limitations should be considered when interpreting the results of this study. First, the retrospective design does not allow us to infer causal relationships among variables. Second, this study relies on administrative data, which may have led to the misclassification of patients with amyloidosis, given the reduced awareness of this clinical entity in the past and the consequent underuse of the corresponding ICD codes. Third, we were unable (given the limitations of the TriNetX platform) to include in the PSM the degree of aortic stenosis, the proportion of valve-in-valve procedures, the main TAVI access, device selection, and left and right ventricular function. Fourth, the distinction between ATTR-CA and AL-CA was not feasible, highlighting areas for future research. In addition, we did not have access to certain important data, such as echocardiographic, cardiac magnetic resonance, or bone scintigraphy results for each patient. Fifth, considering that the TAVI procedure has changed dramatically over the last 2 decades (due to both technical and technological advances), the timing of the procedure might have influenced our risk estimation, although the subgroup analysis did not show a significant difference between the 2 time periods considered (2005-2019 and 2020-2023). Sixth, patients requiring a PM typically exhibit conduction system abnormalities, often identified during routine follow-up. This results in interval-censoring, as the exact onset of the need for a PM is frequently unknown. To accurately analyze the risk of conduction system disease associated with amyloidosis following TAVI, the most rigorous approach would involve determining the precise onset of conduction system disturbances or, alternatively, the use of interval-censored survival analysis to evaluate the risk of PM implantation. The use of traditional survival methods, as applied in the present study, may have introduced bias, potentially leading to inaccurate estimates of the HRs for PM implantation. Lastly, we did not perform a competing risk analysis. However, the cumulative incidence of mortality observed in our study is unlikely to significantly affect the estimates for nonfatal outcomes, as previous studies have shown that such estimates become less reliable when the cumulative incidence of mortality exceeds 20% to 30%.36–38 Nonetheless, future studies using competing risk methods, such as the Fine and Gray model, are encouraged in scenarios with higher mortality risk. In the present study, we considered only sex and not gender, as recommended by the SAGER guidelines.
CONCLUSIONSPatients with CA have a significantly higher risk of adverse events in the first year following TAVI, particularly HF, as well as an increased risk of PM implantation and stroke in the long-term. Further studies are needed to fully understand how amyloidosis affects TAVI outcomes and to investigate the specific role of amyloid fibrils in the deterioration of valve structure.
FUNDINGNo funding was received for the preparation of this article.
ETHICAL CONSIDERATIONSThis retrospective study is exempt from informed consent. The data reviewed are a secondary analysis of existing data, do not involve intervention or interaction with human participants, and are deidentified as per the deidentification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. The process by which the data were deidentified is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule. This formal determination by a qualified expert was refreshed on December 2020. In the present study, we considered only the sex variable and, unfortunately, not the gender variable, as recommended by the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence has not been used in the preparation of this work.
AUTHORS’ CONTRIBUTIONSL. Gerra, T. Bucci: concept, design, manuscript drafting, literature search, statistical analysis and interpretation. All authors: critical revision and final approval of the manuscript. L. Gerra and T. Bucci share first authorship on this manuscript. L. Gerra, T. Bucci, and G.Y.H. Lip are responsible for the data published in this study.
CONFLICTS OF INTERESTThe authors have no conflicts of interest.
- -
Amyloidosis is a progressive disease caused by insoluble misfolded proteins that can accumulate in the heart, increasing myocardial stiffness, damaging the conduction system, and promoting valvular dysfunction.
- -
Amyloidosis is common in patients with aortic stenosis, particularly those undergoing TAVI.
- -
The impact of amyloid fibrils on clinical outcomes post-TAVI remains unclear, with existing studies showing conflicting results.
- -
This study evaluated the 1-year risk of adverse events after TAVI in patients with amyloidosis.
- -
Amyloidosis patients had a higher risk of HF, stroke, and PM implantation, but no differences in AKI or mortality compared with patients without amyloidosis.
- -
Further studies are needed to define the exact role of amyloid fibrils on the myocardium and valve tissue following TAVI.