The impact of preoperative left ventricular (LV) unloading on postoperative outcomes in patients bridged with venoarterial extracorporeal membrane oxygenation (VA-ECMO) to heart transplantation (HT) is unknown. Our aim was to compare posttransplant outcomes in patients bridged to HT with VA-ECMO, with or without the use of different mechanical strategies for LV decompression.
MethodsWe conducted a retrospective analysis of the postoperative outcomes of consecutive HT candidates bridged with VA-ECMO, with or without concomitant LV unloading. Patients were included from 16 Spanish centers from 2010 to 2020. The primary endpoint was 1-year post-HT survival, which was assessed using Cox regression.
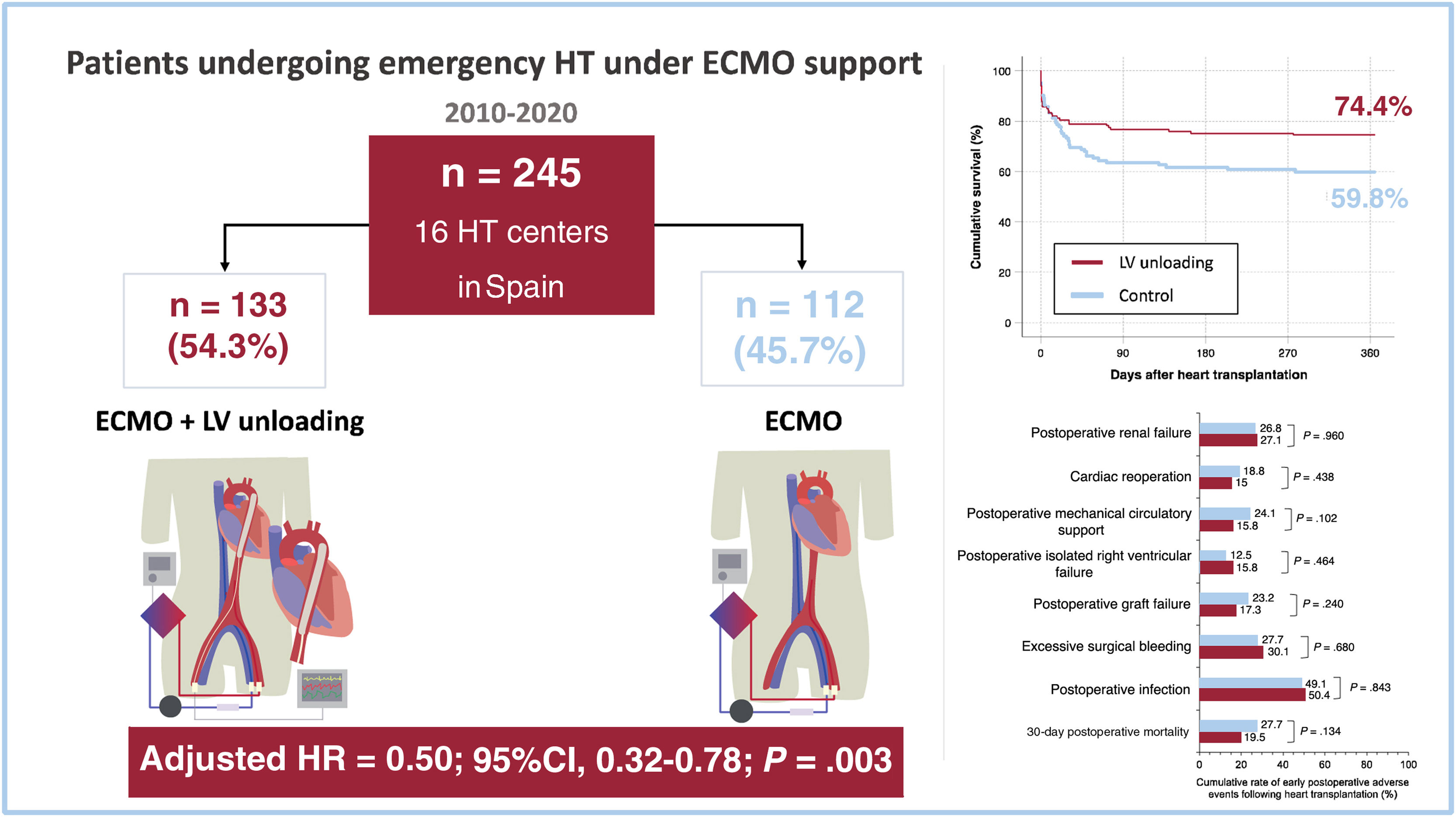
ResultsOverall, 245 patients underwent high-emergency HT while supported with VA-ECMO. A mechanical strategy for LV unloading was used in 133 (54.3%) patients, with the intra-aortic balloon pump being the most commonly used method (n=112; 84.2%). One-year posttransplant survival was 74.4% in the LV unloading group and 59.8% in the control group (P=.025). In multivariate analyses, preoperative LV unloading was independently associated with lower 1-year mortality (adjusted HR, 0.50; 95%CI, 0.32–0.78; P=.003). This association was observed both in patients managed with an intra-aortic balloon pump alone (adjusted HR, 0.52; 95%CI, 0.32–0.84; P=.007) and with other strategies for mechanical LV unloading (adjusted HR, 0.43; 95%CI, 0.19–0.97; P=.042). No significant differences were found between groups regarding other postoperative complications.
ConclusionsPreoperative LV unloading was independently associated with increased 1-year posttransplant survival in candidates bridged with VA-ECMO.
Keywords
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) can be used to bridge patients with cardiogenic shock to heart transplantation (HT). However, the efficacy and safety of this strategy have never been evaluated in randomized clinical trials. Data from multicenter registries indicate that these candidates have an increased risk of early posttransplant mortality.1 Despite this, posttransplant outcomes for patients bridged to HT with VA-ECMO have significantly improved in recent years, likely due to better pretransplant clinical management, more refined candidate selection, and improved timing of transplant surgery.2
Left ventricular (LV) unloading is a major issue in the clinical management of patients on VA-ECMO, as it helps prevent pulmonary congestion and respiratory failure caused by increased systemic afterload. Observational studies have suggested that the use of various mechanical strategies for LV decompression may be associated with improved survival in patients with cardiogenic shock supported by VA-ECMO.3,4 Mechanical methods for LV unloading include atrial septostomy,5 transaortic insertion of a pigtail catheter into the left ventricle,6 surgical venting of the LV,7 left atrium7 or pulmonary artery,8 as well as simultaneous support with an intra-aortic balloon pump (IABP)9 or temporary LV assist devices.10
To our knowledge, the potential beneficial impact of LV unloading on the postoperative outcomes of HT candidates bridged with VA-ECMO has not been demonstrated. A previous small study investigated the use of IABP for this purpose but found no survival advantage when combining IABP with VA-ECMO compared with VA-ECMO alone.11
The aim of this study was to compare posttransplant outcomes in patients bridged to HT with VA-ECMO, with or without different mechanical strategies for LV decompression.
METHODSStudy descriptionClinical data for this study were extracted from the database of a multi-institutional retrospective registry, which included consecutive patients waitlisted for first-time, single-organ, high-emergency HT in 16 Spanish institutions. These patients were treated with various types of temporary mechanical circulatory support devices between January 1, 2010, and December 31, 2020. The study protocol, previously described elsewhere,12 was approved by the Committee for Ethics in Clinical Research of the Autonomous Community of Galicia, Spain, and ratified by the institutional review boards of all participating centers.
Registry data were collected through a retrospective, case-by-case review of medical records for each participant, with local investigators responsible for this task. No external monitoring of the recorded data were performed.
In this manuscript, we analyzed the clinical outcomes of patients included in the registry who underwent HT while supported by VA-ECMO. We compared those who received an additional mechanical strategy for LV unloading with those who did not. Throughout the study period, patients waitlisted under VA-ECMO support were given the highest priority in the Spanish organ donor allocation protocol, referred to as status 0. This priority meant that these candidates had absolute precedence in receiving the first suitable donor organ available within Spain. Specific details regarding the successive modifications of the Spanish organ donor allocation protocol during the study period have been discussed previously.12
As this was an observational, retrospective study, the decision to add mechanical LV unloading to VA-ECMO, including the type and timing, was left to the discretion of the attending clinical team based on local protocols and clinical experience.
Follow-up and outcomesAll patients were followed up from the date of HT up to 1 year after HT, with 1-year posttransplant survival serving as the primary endpoint of the study. We also assessed other relevant adverse clinical outcomes that occurred during the in-hospital postoperative period, including excessive surgical bleeding, cardiac reoperation, postoperative graft dysfunction, postoperative infection, the need for postoperative mechanical circulatory support, and postoperative renal failure requiring dialysis. Specific definitions of all study outcomes are provided in the .
Statistical analysisQualitative variables are presented as the number of patients and percentages, while quantitative variables are expressed as means±standard deviation or medians [interquartile range], as appropriate. Statistical comparisons between groups were conducted using the chi-square test for qualitative variables and the Student t-test or Mann-Whitney test, as appropriate, for quantitative variables.
Kaplan-Meier curves were constructed to graphically represent the cumulative probability of survival during the first year after HT in patients bridged with VA-ECMO alone or with VA-ECMO and LV unloading strategies. The survival functions were compared using the log-rank test.
Multivariable Cox regression was used to adjust for potential confounders in the observed association between LV unloading and 1-year posttransplant survival. A backward stepwise analysis with a P-out criterion >.10 was conducted to identify clinical variables independently associated with the outcome of interest. All baseline clinical variables were considered unless they had more than 10% missing values (bilirubin, albumin, aspartate aminotransferase, pH, PaO2/FiO2 ratio, LV end-diastolic diameter, cardiac index, central venous pressure, capillary wedge pressure, systolic pulmonary artery pressure, diastolic pulmonary artery pressure, mean pulmonary artery pressure, mean transpulmonary gradient).
Variables selected for inclusion in the first step of the backward stepwise process were those that had a univariate association with 1-year posttransplant survival at a P value <.20. Candidate variables entering the multivariable model included recipient age, history of stroke, preoperative infection, preoperative invasive mechanical ventilation, preoperative renal replacement therapy, preoperative vasopressor use, cold ischemia time, preoperative hemoglobin, preoperative creatinine, and mechanical LV unloading.
The final multivariable model from the backward stepwise process was used to estimate the adjusted hazard ratio (HR) for 1-year posttransplant mortality in patients with mechanical LV unloading vs controls. The proportional hazards assumption was verified through graphical representation of Schoenfeld residuals against time (P value for the global test=.820).
Further adjustments were made to validate the results by adding other covariates related to recipients, donors, and devices that were asymmetrically distributed between the study groups to the basic model.
Given the increased use of mechanical LV unloading strategies as an adjunct to VA-ECMO over time and the overall improvement in emergency HT outcomes in recent years, we conducted an exploratory analysis of 1-year post-HT survival in both study groups, stratified by temporal eras. This was done to rule out significant confounding effects of temporal changes on the observed associations. Temporal eras were defined according to historical modifications of the Spanish organ donor allocation protocol, as previously described.12 Era 1 spanned January 2010 to May 2014, Era 2 from June 2014 to May 2017, and Era 3 from June 2017 to December 2020. All statistical analyses were performed using SPSS version 25.
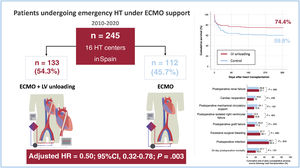
RESULTSPatientsDuring the study period, 245 patients (184 men and 61 women) underwent high-emergency HT while being supported by VA-ECMO at the participating institutions. A mechanical strategy for LV unloading was used in 133 patients (54.3%), who formed the intervention group. The remaining 112 patients (45.7%) were supported with VA-ECMO alone, constituting the control group (figure 1).
Mechanical methods used for LV unloading included IABP support (n=112), left ventricular apical venting (n=12), left atrial venting (n=9), atrial septostomy (n=4), pulmonary artery venting (n=2), temporary left ventricular assist device implantation (n=2), and other or unspecified methods (n=1).
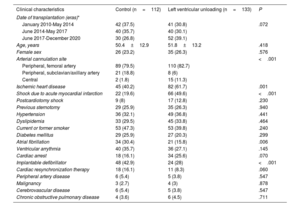
Baseline clinical characteristicsTable 1 shows a comparison of preoperative clinical characteristics of patients bridged to HT on VA-ECMO with or without a mechanical strategy for LV unloading.
Baseline clinical characteristics of study patients
| Clinical characteristics | Control (n=112) | Left ventricular unloading (n=133) | P |
|---|---|---|---|
| Date of transplantation (eras)* | |||
| January 2010-May 2014 | 42 (37.5) | 41 (30.8) | .072 |
| June 2014-May 2017 | 40 (35.7) | 40 (30.1) | |
| June 2017-December 2020 | 30 (26.8) | 52 (39.1) | |
| Age, years | 50.4±12.9 | 51.8±13.2 | .418 |
| Female sex | 26 (23.2) | 35 (26.3) | .576 |
| Arterial cannulation site | <.001 | ||
| Peripheral, femoral artery | 89 (79.5) | 110 (82.7) | |
| Peripheral, subclavian/axillary artery | 21 (18.8) | 8 (6) | |
| Central | 2 (1.8) | 15 (11.3) | |
| Ischemic heart disease | 45 (40.2) | 82 (61.7) | .001 |
| Shock due to acute myocardial infarction | 22 (19.6) | 66 (49.6) | <.001 |
| Postcardiotomy shock | 9 (8) | 17 (12.8) | .230 |
| Previous sternotomy | 29 (25.9) | 35 (26.3) | .940 |
| Hypertension | 36 (32.1) | 49 (36.8) | .441 |
| Dyslipidemia | 33 (29.5) | 45 (33.8) | .464 |
| Current or former smoker | 53 (47.3) | 53 (39.8) | .240 |
| Diabetes mellitus | 29 (25.9) | 27 (20.3) | .299 |
| Atrial fibrillation | 34 (30.4) | 21 (15.8) | .006 |
| Ventricular arrythmia | 40 (35.7) | 36 (27.1) | .145 |
| Cardiac arrest | 18 (16.1) | 34 (25.6) | .070 |
| Implantable defibrillator | 48 (42.9) | 24 (28) | <.001 |
| Cardiac resynchronization therapy | 18 (16.1) | 11 (8.3) | .060 |
| Peripheral artery disease | 6 (5.4) | 5 (3.8) | .547 |
| Malignancy | 3 (2.7) | 4 (3) | .878 |
| Cerebrovascular disease | 6 (5.4) | 5 (3.8) | .547 |
| Chronic obstructive pulmonary disease | 4 (3.6) | 6 (4.5) | .711 |
The data are expressed as No. (%) or mean±standard deviation.
Temporal eras were defined according to changes in the Spanish organ donor allocation protocol, which were described in detail in Barge-Caballero et al.12
Ischemic heart disease and cardiogenic shock related to acute myocardial infarction were more common among patients with LV unloading while atrial fibrillation and previous defibrillator implantation were more common in patients without LV unloading.
Femoral cannulation was the predominant access for arterial cannulation in both study groups (n=199; 81.2%). Among patients in whom alternative arterial cannulation sites were used, central cannulation predominated in the LV unloading group, while subclavian/axillary cannulation predominated in the control group.
The LV unloading group had a higher percentage of patients treated in the most recent era, and a lower proportion of patients with a history of cardiac arrest or cardiac resynchronization therapy, although none of these differences reached statistical significance.
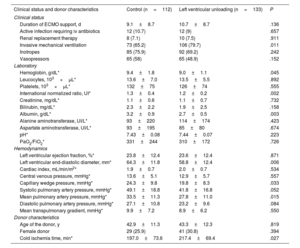
Pretransplant clinical statusTable 2 compares the clinical status, laboratory tests, and hemodynamics of recipients before transplantation. Patients bridged with ECMO and LV unloading were more frequently intubated at the time of HT than those in the control group. No other significant differences were observed between the groups regarding vital supportive therapies, the rate of pretransplant infection, or the duration of ECMO support. Patients with LV unloading also had lower mean serum albumin and international normalized ratio values than those in the control group.
Clinical status of study patients before transplantation and donor characteristics
| Clinical status and donor characteristics | Control (n=112) | Left ventricular unloading (n=133) | P |
|---|---|---|---|
| Clinical status | |||
| Duration of ECMO support, d | 9.1±8.7 | 10.7±8.7 | .136 |
| Active infection requiring iv antibiotics | 12 (10.7) | 12 (9) | .657 |
| Renal replacement therapy | 8 (7.1) | 10 (7.5) | .911 |
| Invasive mechanical ventilation | 73 (65.2) | 106 (79.7) | .011 |
| Inotropes | 85 (75.9) | 92 (69.2) | .242 |
| Vasopressors | 65 (58) | 65 (48.9) | .152 |
| Laboratory | |||
| Hemoglobin, g/dL* | 9.4±1.8 | 9.0±1.1 | .045 |
| Leucocytes, 103×μL* | 13.6±7.0 | 13.5±5.5 | .892 |
| Platelets, 103×μL* | 132±75 | 126±74 | .555 |
| International normalized ratio, UI* | 1.3±0.4 | 1.2±0.2 | .002 |
| Creatinine, mg/dL* | 1.1±0.6 | 1.1±0.7 | .732 |
| Bilirubin, mg/dL* | 2.3±2.2 | 1.9±2.5 | .158 |
| Albumin, g/dL* | 3.2±0.9 | 2.7±0.5 | .003 |
| Alanine aminotransferase, UI/L* | 93±220 | 114±174 | .423 |
| Aspartate aminotransferase, UI/L* | 93±195 | 85±80 | .674 |
| pH* | 7.43±0.08 | 7.44±0.07 | .223 |
| PaO2/FiO2* | 331±244 | 310±172 | .726 |
| Hemodynamics | |||
| Left ventricular ejection fraction, %* | 23.8±12.4 | 23.6±12.4 | .871 |
| Left ventricular end-diastolic diameter, mm* | 64.3±11.8 | 58.8±12.4 | .006 |
| Cardiac index, mL/min/m2* | 1.9±0.7 | 2.0±0.7 | .534 |
| Central venous pressure, mmHg* | 13.6±5.1 | 12.9±5.7 | .557 |
| Capillary wedge pressure, mmHg* | 24.3±9.8 | 19.8±8.3 | .033 |
| Systolic pulmonary artery pressure, mmHg* | 49.1±18.8 | 41.8±16.8 | .052 |
| Mean pulmonary artery pressure, mmHg* | 33.5±11.3 | 27.8±11.0 | .015 |
| Diastolic pulmonary artery pressure, mmHg* | 27.1±10.8 | 23.2±9.6 | .084 |
| Mean transpulmonary gradient, mmHg* | 9.9±7.2 | 8.9±6.2 | .550 |
| Donor characteristics | |||
| Age of the donor, y | 42.9±11.3 | 43.3±12.3 | .819 |
| Female donor | 29 (25.9) | 41 (30.8) | .394 |
| Cold ischemia time, min* | 197.0±73.6 | 217.4±69.4 | .027 |
ECMO, extracorporeal membrane oxygenation; iv, intravenous.
The data are expressed as No. (%) or mean±standard deviation.
Missing values: hemoglobin (n=10), leucocytes (n=4), platelets (n=3), international normalized ratio (n=6), creatinine (n=5), bilirubin (n=31), albumin (n=120), alanine aminotransferase (n=21), aspartate aminotransferase (n=37), pH (n=40), PaO2/FiO2 (n=109), left ventricular ejection fraction (n=23), left ventricular end-diastolic diameter (n=92), cardiac index (n=136), central venous pressure (n=158), capillary wedge pressure (n=166), systolic pulmonary artery pressure (n=152), diastolic pulmonary artery pressure (n=162), mean pulmonary artery pressure (n=148), mean transpulmonary gradient (n=167), cold ischemia time (n=1).
Preoperative hemodynamic parameters were measured in a small proportion of patients. Mean values of LV ejection fraction, LV end-diastolic diameter, pulmonary artery pressure and pulmonary capillary wedge pressure were significantly lower in the LV unloading group than in the control group.
DonorsThe most relevant clinical characteristics of implanted heart donors are detailed in table 2. Mean cold ischemia times were significantly longer in the LV unloading group than in the control group (217.9±69.4 vs 197.0±73.6minutes; P=.032). The sex distribution and mean age of donors were comparable between the 2 groups..
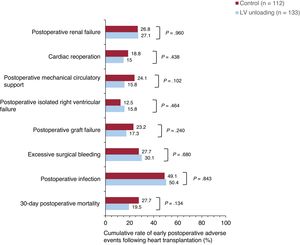
In-hospital postoperative adverse events after transplantationFigure 1 and figure 2 compare major adverse clinical outcomes during the in-hospital postoperative period after HT in both study groups. The cumulative rates of postoperative graft failure and the need for mechanical circulatory support were 17.3% and 15.8% in patients bridged with LV unloading, compared with 23.2% and 24.1% in the control group, respectively. However, these differences did not reach statistical significance (P for postoperative graft failure=.240; P for postoperative need for mechanical circulatory support=.102). The cumulative rates of isolated postoperative right ventricular failure, excessive surgical bleeding, cardiac reoperation, postoperative infection, and postoperative renal failure were comparable between the 2 groups.
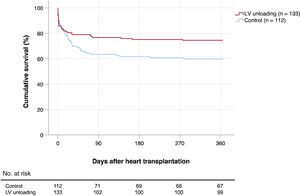
Posttransplant survivalOverall, 79 patients (32.2%) died during the first year after HT. Causes of death included infection (n=28), primary graft dysfunction (n=27), unspecified multiorgan failure (n=9), bleeding (n=8), acute rejection (n=2), stroke (n=2), renal failure (n=1), and respiratory failure (n=1). Kaplan-Meier survival curves are shown in figure 1 and figure 3. The cumulative 1-year posttransplant survival was 74.4% in the LV unloading group (34 deaths) and 59.8% in the control group (45 deaths). According to the univariate log-rank test, patients bridged to HT with LV unloading had significantly higher 1-year posttransplant survival than those in the control group (P=.025).
Among male candidates, the 1-year posttransplant survival was 76.5% in those treated with LV unloading vs 62.8% in those without unloading (P=.042). Among female candidates, the 1-year posttransplant survival was 68.6% in those treated with LV unloading and 50% in those without unloading (P=0.142)
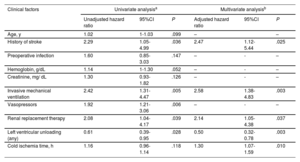
Multivariate backward stepwise Cox regression analyses identified 5 clinical factors associated with the risk of 1-year mortality following HT in the study cohort (table 3): history of previous stroke (adjusted HR, 2.47; 95% confidence interval [95%CI], 1.12-5.44; P=.025), cold ischemia time (adjusted HR, 1.30; 95%CI, 1.07-1.59; P=.010), pretransplant renal replacement therapy (adjusted HR, 2.14; 95%CI, 1.05–4.38; P=.037), pretransplant invasive mechanical ventilation (adjusted HR, 2.58; 95%CI, 1.38–4.83; P=.003) and pretransplant LV unloading (adjusted HR, 0.50; 95%CI, 0.32-0.78; P=.003). The strength of the association between pretransplant LV unloading and 1-year mortality was similar in men (adjusted HR 0.48, 95%CI 0.28–0.83) and women (adjusted HR 0.49; 95%CI 0.21-1.12); however, in the latter group, the association did not reach statistical significance, likely due to the limited sample size.
Clinical factors associated with 1-year posttransplant mortality: univariate and multivariate Cox's regression analyses
| Clinical factors | Univariate analysisa | Multivariate analysisb | ||||
|---|---|---|---|---|---|---|
| Unadjusted hazard ratio | 95%CI | P | Adjusted hazard ratio | 95%CI | P | |
| Age, y | 1.02 | 1-1.03 | .099 | – | – | |
| History of stroke | 2.29 | 1.05-4.99 | .036 | 2.47 | 1.12-5.44 | .025 |
| Preoperative infection | 1.60 | 0.85-3.03 | .147 | – | - | – |
| Hemoglobin, g/dL | 1.14 | 1-1.30 | .052 | – | - | – |
| Creatinine, mg/ dL | 1.30 | 0.93-1.82 | .126 | – | - | – |
| Invasive mechanical ventilation | 2.42 | 1.31-4.47 | .005 | 2.58 | 1.38-4.83 | .003 |
| Vasopressors | 1.92 | 1.21-3.06 | .006 | – | - | – |
| Renal replacement therapy | 2.08 | 1.04-4.17 | .039 | 2.14 | 1.05-4.38 | .037 |
| Left ventricular unloading (any) | 0.61 | 0.39-0.95 | .028 | 0.50 | 0.32-0.78 | .003 |
| Cold ischemia time, h | 1.16 | 0.96-1.14 | .118 | 1.30 | 1.07-1.59 | .010 |
95%CI, 95% confidence interval.
The inverse association between mechanical LV unloading and 1-year mortality was observed in both patients managed with an IABP alone (adjusted HR, 0.52; 95%CI, 0.32-0.84; P=.007) and those managed with other strategies for mechanical LV unloading (adjusted HR, 0.43; 95%CI, 0.19–0.97; P=.042).
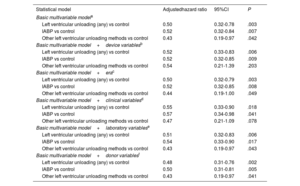
Further multivariable adjustments were performed to assess potential confounding bias by adding other covariables related to temporal eras and characteristics of devices, recipients, and donors that were considered potential confounders. However, no relevant changes in the observed statistical associations were noted (table 4).
Statistical associations between left ventricular unloading and 1-year posttransplant mortality: results based on different multivariable models
| Statistical model | Adjustedhazard ratio | 95%CI | P |
|---|---|---|---|
| Basic multivariable modela | |||
| Left ventricular unloading (any) vs control | 0.50 | 0.32-0.78 | .003 |
| IABP vs control | 0.52 | 0.32-0.84 | .007 |
| Other left ventricular unloading methods vs control | 0.43 | 0.19-0.97 | .042 |
| Basic multivariable model+device variablesb | |||
| Left ventricular unloading (any) vs control | 0.52 | 0.33-0.83 | .006 |
| IABP vs control | 0.52 | 0.32-0.85 | .009 |
| Other left ventricular unloading methods vs control | 0.54 | 0.21-1.39 | .203 |
| Basic multivariable model+erac | |||
| Left ventricular unloading (any) vs control | 0.50 | 0.32-0.79 | .003 |
| IABP vs control | 0.52 | 0.32-0.85 | .008 |
| Other left ventricular unloading methods vs control | 0.44 | 0.19-1.00 | .049 |
| Basic multivariable model+clinical variablesd | |||
| Left ventricular unloading (any) vs control | 0.55 | 0.33-0.90 | .018 |
| IABP vs control | 0.57 | 0.34-0.98 | .041 |
| Other left ventricular unloading methods vs control | 0.47 | 0.21-1.09 | .078 |
| Basic multivariable model+laboratory variablese | |||
| Left ventricular unloading (any) vs control | 0.51 | 0.32-0.83 | .006 |
| IABP vs control | 0.54 | 0.33-0.90 | .017 |
| Other left ventricular unloading methods vs control | 0.43 | 0.19-0.97 | .043 |
| Basic multivariable model+donor variablesf | |||
| Left ventricular unloading (any) vs control | 0.48 | 0.31-0.76 | .002 |
| IABP vs control | 0.50 | 0.31-0.81 | .005 |
| Other left ventricular unloading methods vs control | 0.43 | 0.19-0.97 | .041 |
95%CI, 95% confidence interval; IABP, intra-aortic balloon pump.
Adjustment covariables: history of stroke, preoperative mechanical ventilation, preoperative renal replacement therapy, cold ischemia time.
Adjustment covariables: history of stroke, preoperative mechanical ventilation, preoperative renal replacement therapy, cold ischemia time, type of arterial cannulation, duration of extracorporeal membrane oxygenation support before transplantation.
Adjustment covariables: history of stroke, preoperative mechanical ventilation, preoperative renal replacement therapy, cold ischemia time, era of transplantation.
Adjustment covariables: history of stroke, preoperative mechanical ventilation, preoperative renal replacement therapy, cold ischemia time, age, sex, cardiogenic shock due to acute myocardial infarction, history of atrial fibrillation, history of cardiac arrest, implantable cardiac device.
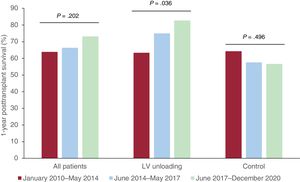
Figure 4 shows a stratified analysis of 1-year posttransplant survival rates in the study population according to temporal eras. In the whole cohort, 1-year posttransplant survival increased from 63.9% in Era 1 to 66.3% in Era 2 and 73.9% in Era 3; however, this trend did not reach statistical significance (P=.240).
The 1-year posttransplant survival rates of patients bridged with LV unloading increased significantly over time (Era 1=63.4%; Era 2=75%; Era 3=82.7%; P=.036) and were higher than those of the control group, which remained unchanged (Era 1=64.3%; Era 2=57.5%; Era 3=56.7%; P=.496).
DISCUSSIONIn our study based on a multi-institutional Spanish cohort of patients bridged to HT under VA-ECMO support, mechanical LV unloading, independently of its type, was associated with significantly higher 1-year posttransplant survival. To the best of our knowledge, this is the first study to suggest that the use of LV unloading may positively influence posttransplant outcomes in these patients.
VA-ECMO is widely used in patients with cardiogenic shock as a potential bridge to myocardial recovery or heart replacement therapies, such as HT or LV assist device implantation.13 However, VA-ECMO support is associated with several detrimental hemodynamic effects, including increased LV afterload, inadequate opening of the aortic valve, elevation of LV end-diastolic pressure, LV dilation and worsening ventricular function, myocardial ischemia, pulmonary edema, and thrombus formation in the ventricle.14,15
Various mechanical methods for LV unloading can be used to mitigate the adverse hemodynamic consequences of VA-ECMO physiology, including various surgical or percutaneous venting strategies of the left heart chambers, as well as concomitant support with adjunctive mechanical devices such as IABP or percutaneous LV assist devices. In our cohort, more than half of the patients were managed with LV unloading, mostly through the insertion of an IABP, in line with other studies.3
A meta-analysis of observational studies suggested that LV unloading may be associated with improved survival in patients with cardiogenic shock supported by VA-ECMO.16–18 However, a recent randomized clinical trial19 failed to demonstrate a significant impact of an early routine strategy of LV unloading using a transeptal left atrial cannula. Remarkably, the crossover rate was high in this trial, with almost half of the patients in the control group managed with LV unloading at some point during the follow-up period.19
We acknowledge that, in our cohort, patients managed with or without LV unloading had different baseline clinical characteristics, and consequently, the observed influence of the combined support strategy on posttransplant outcomes might be affected by confounding bias. However, it should be noted that the clinical profile of patients receiving LV unloading was not necessarily indicative of a lower risk compared with that in the control group. Indeed, the unloaded group showed higher rates of cardiogenic shock due to acute myocardial infarction, higher rates of invasive mechanical ventilation at the time of transplant surgery, and longer cold ischemia times. Even so, posttransplant survival was significantly higher in the unloaded group. Moreover, the association between preoperative LV unloading and increased posttransplant survival remained statistically significant after extensive multivariable adjustments to control for potential confounders. Interestingly, posttransplant survival rates in the interventional group were similar in patients treated with an IABP or other types of LV mechanical unloading. Previous studies could not demonstrate a clear survival advantage of alternative methods for LV unloading during VA-ECMO support, such as Impella, over simpler concomitant IABP therapy.20
Mechanical LV unloading has several positive pathophysiological effects that may lead to clinical benefits for patients supported with VA-ECMO. For example, concomitant IABP support has been associated with a significant reduction in pulmonary artery occlusion pressure and LV dimensions, as well as a significant increase in pulse pressure.21 Similar effects were observed in the small cohort of patients in our study who had available hemodynamic data; LV unloading was associated with lower LV diameters, lower capillary wedge pressure and lower mean pulmonary pressure. This preoperative hemodynamic improvement could impact patient prognosis, consistent with findings in other cardiac surgery.22 In the specific bridge-to-transplant scenario, there is a pathophysiological rationale to hypothesize that reducing pulmonary congestion and pulmonary pressures in candidates waiting for a donor heart might result in better posttransplant outcomes, mainly by decreasing the risk of early failure of the donor heart. In our study, patients bridged with LV unloading showed numerically lower rates of early postoperative graft dysfunction and the need for mechanical circulatory support; however, this association was not statistically significant.
In our cohort, 1-year posttransplant survival was acceptable, considering the critical status of the treated population. Historically, posttransplant outcomes of Spanish transplant candidates bridged on VA-ECMO were significantly inferior to those of candidates bridged with other modes of mechanical circulatory support.23 However, the posttransplant outcomes of VA-ECMO-bridged patients experienced secular improvement in the study cohort, and the increasing rates of use of ancillary mechanical strategies for LV unloading might have played an important role in this context. Indeed, the 1-year posttransplant survival of patients bridged to urgent HT with combined VA-ECMO support and LV unloading reached 82%, a figure that is quite close to that observed in other international high-volume centers.24
LimitationsThis study has several limitations. As a retrospective investigation, it may be subject to various sources of bias, including selection, information, and confounding biases. Therefore, its results should be considered hypothesis-generating only. A strength of this investigation is its inclusion of all activity from Spanish centers that maintained an active adult HT program during the evaluated period. However, this also means there may be potential differences in selection protocols and therapeutic management among centers that were not controlled for in the analysis, and clinical events were adjudicated by local investigators rather than by an independent committee. While our results can be directly applied to Spain, caution is warranted when applying them to other countries with organ-sharing donor systems that may differ from the Spanish system.
We acknowledge that the characterization of the study population could have been improved if additional important clinical variables, such as the vasoactive-inotropic score or the Society of Cardiovascular Angiography and Intervention stages, had been collected; however, this information was not available for the study. Moreover, the number of missing values in hemodynamic variables was too high to allow us to draw reliable conclusions. Additionally, we cannot provide specific information regarding the type and duration of posttransplant mechanical circulatory support for those patients who required it, so no conclusions can be drawn about its potential impact on study outcomes.
Finally, while significant effort has been made to adjust for the most relevant potential confounders, some variables that could affect these differences may be missing. Despite this, we have collected a large number of variables to minimize the influence of possible confounding factors.
CONCLUSIONSOur study suggests that LV unloading in patients managed with VA-ECMO as a bridge to emergent HT might be associated with improved 1-year posttransplant survival; however, the reasons that might explain this finding are not fully clear. Further clinical and mechanistic studies are necessary to confirm this novel hypothesis and to provide pathophysiological information that supports the potential benefits of LV unloading in the bridge-to-transplant setting before specific therapeutic recommendations can be made in this regard.
FUNDINGThe ASIS-TC study was funded by the Fundación Mutua Madrileña (Madrid, Spain) through 2 competitive research grants (Ayudas para Investigación en Salud, X and XIV annual announcements, years 2014 and 2018), gained by the first author of this manuscript (E. Barge-Caballero).
ETHICAL CONSIDERATIONSThe study protocol was first approved by the Committee for Ethics in Clinical Investigation of the Autonomous Community of Galicia (Spain) and subsequently ratified by the institutional review boards of all hospitals participating in the study. Given the retrospective nature of the study, investigators obtained a waiver from the ethics committee to avoid the necessity of obtaining written informed consent from study participants. All clinical data collected in the study were pseudonymized, ensuring that study participants could not be identified by third parties. The study took into account sex and gender variables in accordance with SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this paper.
AUTHORS’ CONTRIBUTIONSD. Enríquez-Vázquez and E. Barge-Caballero contributed equally to the manuscript and are the first authors. D. Enríquez-Vázquez contributed to the conceptualization, manuscript drafting, and data collection. E. Barge-Caballero contributed to the conceptualization, manuscript drafting, data collection, funding acquisition, statistical analysis, coordination, and supervision. M.G. Crespo-Leiro contributed to funding acquisition, data collection, manuscript editing, coordination, and supervision. J. Muñiz contributed to funding acquisition, statistical analysis, and manuscript editing. All other authors contributed to data collection and manuscript editing.
CONFLICTS OF INTERESTNothing to disclose.
- •
VA-ECMO may be used as a direct bridge to emergency heart transplantation in certain selected candidates who are critically ill.
- •
Mechanical left ventricular unloading is frequently used as an adjunctive therapy in patients supported with VA-ECMO to prevent the development of left ventricular distension and pulmonary congestion, which are known consequences of increased left ventricular afterload in these patients.
- •
Despite some evidence supporting the clinical benefits of mechanical left ventricular unloading in patients with cardiogenic shock treated with VA-ECMO, its potential impact in the specific setting of bridge-to-transplantation remains unknown.
- •
Our study suggests that the concomitant use of mechanical left ventricular unloading in patients bridged to emergency heart transplantation with VA-ECMO might be associated with improved posttransplant outcomes.