Transcatheter aortic valve implantation (TAVI) is now the method of choice for treating patients with symptomatic aortic valve disease, who are deemed by the Heart Team to be at high or inoperable risk.
Using the Edwards Sapien system, the Partner Study demonstrated a similar mortality rate to surgery at 30 days (3.4% vs 6.5%; P = .07) and at 1 year (24.2% vs 26.8%; P = .44).1 The Medtronic CoreValve system went further to demonstrate a significantly higher survival rate at 1 year vs surgery (14.2% vs 19.1%; P = .04).2 Challenges such as vascular complications,1–3 paravalvular regurgitation,4–7 need for permanent pacing8,9 and stroke,10 however, existed with these early-generation devices. Although patient selection and operator experience could have accounted for some of these events, device-related challenges are thought to have been contributing factors.
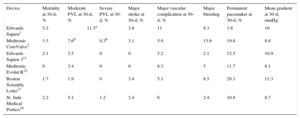
The Edwards Sapien 3 and the Commander delivery system, with its 14-16F e-sheath are the latest iterations to the Edwards TAVI system. The Sapien 3 valve has an external skirt, aiming to improve paravalvular regurgitation. The Commander delivery system, with its double flexion capability and the reduced sheath profile, is designed to improve valve delivery and deployment. Early study results suggest improvements in outcome compared with its predecessors.11 The Medtronic Evolut R valve, with the Enveo delivery catheter and InLine sheath are the current generation of the TAVI system from Medtronic.12 The supra-annular valve is more conformable, aiming to reduce paravalvular regurgitation by better adherence to the surrounding tissue. The new delivery system enables the valve to be fully repositionable and recapturable prior to full release. The built-in InLine sheath allows for the whole system to be inserted into a patient sheathless, thus, reducing the overall profile of the system, similar to the outer diameter of a 14F sheath; this makes the Evolut R the lowest-profiled commercially available TAVI system. Early CE mark study demonstrates the overall event rates for key parameters with the Evolut R to be improved compared with CoreValve.12 Other next-generation devices (Boston Scientific Lotus and St Jude Medical Portico TAVI systems) have shown similar good results in early studies.13,14 The Table summarizes some of the key outcomes from the next-generation TAVI systems.
How these new devices function in the ‘real world’ remains to be seen. In the article published in Revista Española de Cardiología, Perrin et al.15 share their single-center experience with the Medtronic Evolut R system. A total of 71 patients were treated with the new system, accounting for 85.5% of the total TAVI procedures in their institution in a 14-month period. Clinical endpoints were independently adjudicated to Valve Academic Research Consortium (VARC)-2 criteria. Although the CE mark study, with 60 patients treated, had no mortality at 30 days,12 the 2.8% observed in this ‘all-comer’ patient series was similar to recent published outcomes (Table). A total of 4 patients required either a second valve or a surgical valve–these appear to be related to technical/procedural challenges, suggesting a learning curve requirement for new devices, although it is not clear when these were encountered in their experience.
Summary of 30-Day Outcomes Between Early-generation and Current-generation TAVI Devices
| Device | Mortality at 30-d, % | Moderate PVL at 30-d, % | Severe PVL at 30-d, % | Major stroke at 30-d, % | Major vascular complication at 30-d, % | Major bleeding | Permanent pacemaker at 30-d, % | Mean gradient at 30-d, mmHg |
|---|---|---|---|---|---|---|---|---|
| Edwards Sapien1 | 5.2 | 11.5a | 3.8 | 11 | 9.3 | 3.8 | 10 | |
| Medtronic CoreValve2 | 3.3 | 7.6b | 0.3b | 3.1 | 5.9 | 13.6 | 19.8 | 8.8 |
| Edwards Sapien 311 | 2.1 | 3.5 | 0 | 0 | 5.2 | 2.1 | 12.5 | 10.9 |
| Medtronic Evolut R12 | 0 | 3.4 | 0 | 0 | 8.3 | 5 | 11.7 | 8.1 |
| Boston Scientific Lotus13 | 1.7 | 1.9 | 0 | 3.4 | 5.1 | 8.5 | 29.3 | 11.3 |
| St. Jude Medical Portico14 | 2.2 | 5.1 | 1.2 | 2.4 | 6 | 2.4 | 10.8 | 8.7 |
PVL, paravalvular leak.
Perrin et al. report that resheathing was performed in 21% of patients to optimize positioning, resulting in good 30-day paravalvular regurgitation rates (1.6% with moderate).15 The permanent pacing rates, however, were higher than the CE mark study (23.9% vs 11.7%). The authors believed that this could be due to lower final positioning of the valve compared with the CE study. Of importance, as with the CE study, resheathing was not associated with stroke occurrence, and the rate of major stroke observed by Perrin et al. was low at 1.4% (n = 1). Major vascular complications were observed in 8.3% of the CE mark study patients compared with a lower rate (4.2%) observed by Perrin et al. These figures are an improvement, compared with 5.9% as seen with Medtronic CoreValve2 and 11% with Edwards Sapien studies.1
The VARC-2 definition of device success, requires a composite of: a) absence of procedural mortality; b) correct position of a single prosthetic valve; and c) intended performance of the prosthetic valve (no prosthetic-patient mismatch [PPM], mean valve gradient < 20mmHg or peak velocity < 3 m/sec, and no moderate or severe prosthetic regurgitation). Failure to meet any 1 of these 3 parameters will categorize the device a failure. Device success of 90.1% was reported by Perrin et al., which was higher than that reported in the Evolut R CE study (78.6%).12 The device success rate observed in the CE study was lower than expected and was triggered predominantly by the presence of calculated PPM in 9 patients and unavailable data on a further 5 patients. The challenges of calculating PPM using transthoracic echocardiography, rather than transesophageal echocardiography, is well recognized. It is not clear from the article whether PPM was calculated; nevertheless, the findings of Perrin et al. are reassuring and confirm that the CE mark findings were somewhat artefactual, triggered by the PPM dataset. The 30-day mean gradient reported by Perrin et al. was 7.7 ± 4.1mmHg, which compares favorably with the CE mark study of 8.1 ± 3.3mmHg.
Patients enrolled in the early first-in-man or CE mark studies tend to be controlled by inclusion and exclusion criteria, which tend to select better TAVI candidates for study enrolment. Nevertheless, improvements in key outcome parameters are evident when comparing studies using early-generation devices and next-generation devices (Table). Although some of these improvements could be attributed to better patient selection, operator experience and use of cardiac computed tomography, technological advances have impacted positively on clinical outcome. Equally important are how these results translate to real world experience, and to this end, the results published by Perrin et al. with the Evolut R system adds to our knowledge and understanding of how next-generation TAVI systems impact on our patients. Despite these technological advances, operator diligence and care at the pre-, peri- and postprocedure stages will be extremely important if we are to harness the potential advantages of the next-generation TAVI systems.16
CONFLICTS OF INTERESTG. Manoharan has received consultant fees and honoraria from Medtronic, St Jude Medical and Boston Scientific.