The number of pacemakers implanted continues to increase as a result of increased life expectancy. According to the 2013 Spanish Pacemaker Registry,1 the number of first implants per million inhabitants was 567. Despite improved technology and more extensive experience, complications related to pacemaker implantation are still significant, particularly in relation to transvenous leads and the pacemaker pocket.2
Single-component leadless pacemaker systems have been developed to reduce complications. In these systems, the pacemaker and lead are integrated in a single unit, thereby eliminating the risks associated with the leads, pacemaker pocket, and connections.3
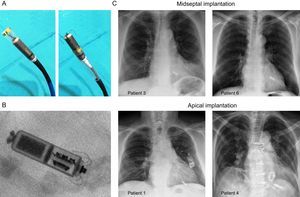
In the present letter, we report our initial experience in a single center with implantation of the Micra transcatheter pacemaker system (Medtronic Ibérica, S.A.), approved for clinical use in Europe in June 2015. The device consists of an 8-cm3 capsule, measuring 25.9×6.7mm, designed to provide bipolar VVI-mode cardiac pacing of the right ventricle, with an estimated longevity of 9.6 years. The device also has automated pacing capture threshold management and can be used in magnetic resonance imaging studies without any body restrictions in 1.5 and 3 Tesla systems. The pacemaker is affixed to the ventricular endocardium by 4 self-expanding electrically inert nitinol tines. The device is positioned with the pacemaker and unexpanded tines at the distal end of a steerable catheter, which lies inside an introducer (23-F internal diameter and 27-F external diameter), inserted via the femoral vein. Before the pacemaker is definitively released from the catheter, and once it is positioned in the ventricle, the operator must confirm that the electrical parameters are appropriate and that the system is well affixed to the endocardium by a fluoroscopically guided pull-and-hold test. If the device is not properly affixed or the electrical parameters are deficient, the pacemaker can be removed and the implantation procedure repeated.
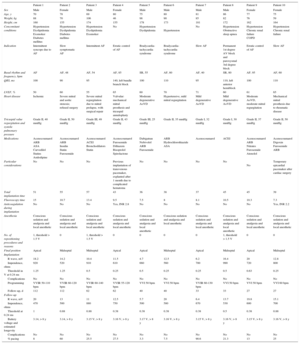
The Micra pacemaker was implanted in 10 patients with a standard indication for a permanent pacemaker and with a clinical profile and indication appropriate for VVI pacing. In this initial phase, patients without their own cardiac rhythm were excluded. Prior to implantation, the patients and family members were informed of the characteristics of the new system and the more limited clinical experience with respect to conventional systems. Informed consent was obtained. The general characteristics of the patients and basic data on the procedure are summarized in the Table. The mean age of the patients was 77.1±5.1 years, 6 were men and 4 were women, and 8 had permanent atrial fibrillation as the basal rhythm and 2 had sinus rhythm. The Micra pacemaker was successfully implanted in 10 patients (Figure) by the same operator (M. P.), who had extensive experience in placement of conventional implantable cardiac devices and in electrophysiological procedures with femoral venous and arterial approaches. The first fixation attempt was definitive in 7 patients. In the remaining 3, repositioning was required, although in all patients only 1 further attempt was needed due to deficient electrical parameters after the initial implantation. The mean values of the R wave and impedance were 12.7±4.8mV and 739±161 ohms, respectively. The mean duration of the implantation procedure was 44.6±7.5minutes, while fluoroscopy lasted 10.03±2.5minutes. No patients experienced sustained arrhythmias or blocks during implantation. The femoral puncture site was closed with a Figure 8 suture, with no incidents of note (2 of the patients continued anticoagulant treatment with acenocoumarol during implantation). The clinical and electrical data are summarized in the Table. Of note is the absence of complications requiring intervention and the low rate of repositioning even though these were the first implantations of the new device. At follow-up (mean duration, 55±33 days, range 27-112 days), thresholds (all = 1V at a pulse width of 0.24ms), R wave (13.4±5.1mV), and pacing impedance (633±138 ohms) were very stable.
Patient Characteristics and Implantation and Follow-up Data
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Male | Male | Male | Male | Female | Female |
| Age, y | 70 | 78 | 69 | 80 | 75 | 80 | 81 | 86 | 77 | 75 |
| Weight, kg | 88 | 70 | 106 | 46 | 98 | 90 | 85 | 82 | 76 | 59 |
| Height, cm | 160 | 155 | 174 | 155 | 178 | 173 | 181 | 172 | 162 | 164 |
| Concomitant conditions | Hypertension Dyslipidemia Exsmoker Diabetes mellitus | Hypertension Dyslipidemia Diabetes mellitus | Hypertension Dyslipidemia Exsmoker | No | Hypertension Dyslipidemia | Hypertension | No | Hypertension Obstructive sleep apnea | Hypertension Chronic renal failure COPD | Hypertension Chronic renal failure |
| Indication | Intermittent syncope due to AF | Slow symptomatic AF | Intermittent AF | Erratic control of AF | Bradycardia-tachycardia syndrome | Bradycardia-tachycardia syndrome | Slow AF | Permanent 1st degree AV block and paroxysmal 3rd degree block | Erratic control of AF | Slow AF |
| Basal rhythm and frequency, bpm | AF | AF, 48 | AF, 54 | AF, 85 | SR, 55 | AF, 80 | AF, 40 | SR, 80 | AF, 95 | AF, 60 |
| QRS, ms | 100 | 90 | 95 | 140, left bundle branch block | 100 | 110 | 95 | 110, left anterior hemiblock | 100 | 110 |
| LVEF, % | 50 | 60 | 55 | 65 | 60 | 70 | 71 | 60 | 61 | 65 |
| Heart disease | Ischemic | Severe mitral and aortic stenosis; refused surgery | Severe mitral regurgitation due to mitral prolapse, with surgical repair | Valvular: mechanical mitral prosthesis and tricuspid annuloplasty | Moderate degenerative AoVD | Hypertensive, mild mitral regurgitation | Mild degenerative AoVD | Mild degenerative AoVD | Moderate AoVD, moderate mitral regurgitation | Mechanical mitral prosthesis due to rheumatic disease |
| Tricuspid value regurgitation and systolic pulmonary pressure | Grade II, 40 mmHg | Grade II, 50 mmHg | Grade III, 49 mmHg | Grade II, 43 mmHg | Grade III, 25 mmHg | Grade II, 35 mmHg | Grade I, 32 mmHg | Grade I, 30 mmHg | Grade II, 37 mmHg | Grade II, 50 mmHg |
| Medications | Acenocoumarol ARB ASA Carvedilol Statins Amlodipine | Acenocoumarol ARB Insulin Statin Furosemide | Acenocoumarol ACEI Bronchodilators Statin | Acenocoumarol Furosemide Diltiazem Bisoprolol Spirolactone | Dabigatran Nebivolol ARB Furosemide | ARB Hydrochlorothiazide ASA | Acenocoumarol | ACEI | Acenocoumarol ARB Nitrates Furosemide Atenolol | Acenocoumarol Digoxin Furosemide ARB |
| Particular considerations | No | No | No | Previous implantation of transvenous pacemaker, explanted after 1 month due to complicated hematoma | No | No | No | No | No | Temporary epicardial pacemaker after cardiac surgery |
| Total implantation time | 51 | 55 | 57 | 43 | 36 | 38 | 37 | 45 | 45 | 39 |
| Fluoroscopy time | 15 | 10.7 | 13.4 | 9.5 | 7.5 | 8 | 8.1 | 10.5 | 10.3 | 7.3 |
| Anticoagulation during implantation | No | No | No | Yes, INR 2.8 | No | No | No | No | No | Yes, INR 2.2 |
| Anesthesia | Conscious sedation and analgesia and local anesthetic | Conscious sedation and analgesia and local anesthetic | Conscious sedation and analgesia and local anesthetic | Conscious sedation and analgesia and local anesthetic | Conscious sedation and analgesia and local anesthetic | Conscious sedation and analgesia and local anesthetic | Conscious sedation and analgesia and local anesthetic | Conscious sedation and analgesia and local anesthetic | Conscious sedation and analgesia and local anesthetic | Conscious sedation and analgesia and local anesthetic |
| No. of repositioning procedures and reasons | 1, threshold > 1.5 V | 0 | 1, threshold > 1.5 V | 0 | 0 | 0 | 0 | 1, threshold > 1.5 V | 0 | 0 |
| Final position | Apical | Midseptal | Midseptal | Apical | Apical | Midseptal | Apical | Midseptal | Apical | Midseptal |
| Implantation | ||||||||||
| R wave, mV | 18.2 | 14.2 | 10.4 | 11.5 | 4.7 | 12.5 | 6.2 | 16.4 | 20 | 12.8 |
| Impendence, ohms | 920 | 520 | 910 | 610 | 660 | 560 | 700 | 990 | 720 | 800 |
| Threshold in V at 0.24 ms | 1.25 | 1.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.25 | 0.5 | 0.63 | 0.25 |
| Complications | No | No | No | No | No | No | No | No | No | No |
| Programming | VVIR 50-110 bpm | VVIR 60-120 bpm | VVIR 60-140 bpm | VVIR 55-120 bpm | VVI 50 bpm | VVI 50 bpm | VVIR 60-130 bpm | VVI 50 bpm | VVI 50 bpm | VVI 60 bpm |
| Follow-up, d | 112 | 112 | 62 | 62 | 40 | 40 | 33 | 33 | 27 | 27 |
| Follow-up | ||||||||||
| R wave, mV | 20 | 13 | 11 | 12.5 | 5.7 | 20 | 6.4 | 13.7 | 19.8 | 15.1 |
| Impendence, ohms | 470 | 500 | 880 | 750 | 500 | 580 | 670 | 530 | 600 | 700 |
| Threshold at 0.24 ms | 1 | 0.88 | 0.88 | 0.38 | 0.38 | 0.38 | 0.38 | 0.5 | 0.38 | 0.88 |
| Battery voltage and estimated longevity | 3.14, > 8 y | 3.14, > 8 y | 3.15 V, > 8 y | 3.16 V, > 8 y | 3.17 V, > 8 y | 3.16 V, > 8 y | 3.15 V, > 8 y | 3.16 V, > 8 y | 3.15 V, > 8 y | 3.16 V, > 8 y |
| Complications | No | No | No | No | No | No | No | No | No | No |
| % pacing | 8 | 60 | 25.5 | 27.5 | 3.3 | 7.5 | 90.6 | 21.3 | 13 | 25 |
ACEI, angiotensin converter enzyme inhibitor; AF, atrial fibrillation; AoVD, aortic valve disease; ARB, angiotensin receptor antagonists; ASA, acetylsalicylic acid; bpm, beats per minute; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; SR, sinus rhythm; V, volts.
A: Image of completely hidden (left) and partially released (right) device in the steerable implantation catheter. B: Radiological detail of the implanted pacemaker in patient 4. C: Posteroanterior chest X-ray in 2 patients with midseptal position and 2 patients with apical position.
The initial results in terms of electrical performance and safety of the implantation procedure for the Micra transcatheter pacemaker are promising in the series presented here. However, to date there are no studies in humans comparing this new system with conventional pacemaker systems. The largest study reported is the Micra Transcatheter Pacing Study. This was a prospective, multicenter, noncomparative study in which the Micra system was successfully implanted in 719 of 725 attempts (99.2%).4 Major complications were reported in 25 patients, with no cases of systemic infection or dislodgement of the pacemaker. The electrical performance at 6 months of follow-up, assessed in 297 patients, was very satisfactory, with a mean pacing capture threshold of 0.54V at 0.24ms, an R-wave of 15.3mV, and an estimated longevity of 12.5 years (94% of devices had a projected longevity of more than 10 years). This performance was similar to that of modern conventional pacemakers.
Future studies will provide information on the long-term safety and efficacy of this new permanent pacing system. These studies should also aim to confirm the potential benefits of this device compared with conventional lead-based devices.
Conflicts of interestM. Pachón is a proctor for the Micra device. M.A. Arias is member of the editorial board of Revista Española de Cardiología.