Implantable cardioverter defibrillators (ICDs) have proven effective for the prevention of sudden death in numerous clinical trials. The required transvenous implantation of the pacing leads is a key limitation of these devices, being associated with frequent periprocedural and follow-up complications. To avoid these problems, a completely subcutaneous defibrillation system has been developed.1 Nonetheless, this device was introduced only recently in Spain2 and current experience is limited. The subcutaneous ICD has 2 main components, a pulse generator (S- ICDTM, SQ-RX 1010, Boston Scientific) that weighs 145g, has an estimated longevity of 5 years, and delivers 80-J shocks with up to 30seconds of postshock pacing, and a lead (Q-Trak 3010, Boston Scientific) for detection and defibrillation. An 8-cm shocking coil is located between 2 sensing electrodes integrated into the lead, which is placed parallel to the sternum in the subcutaneous tissue.1
We present an initial experience with subcutaneous ICD implantation in 8 consecutive patients, representing the largest series to date treated with these new devices in Spain. Because the system cannot provide permanent pacing, we began our experience by selecting patients whose demographic profile and underlying heart condition indicated a low probability, a priori, of requiring pacing in the near future, whether it be permanent antibradycardia pacing, cardiac resynchronization therapy, or pacing to terminate sustained reentrant ventricular tachycardia. The general characteristics of the patients treated are shown in Table 1. Patients and their families were informed of the advantages and disadvantages of this type of therapy and signed a consent form to undergo the procedure. In addition, all patients underwent prior electrocardiographic screening, in which their suitability for subcutaneous ICD implantation was established when at least 1 of the 3 ECG leads that correspond to each of the possible detection vectors of the device was found to be satisfactory (Table 2).1
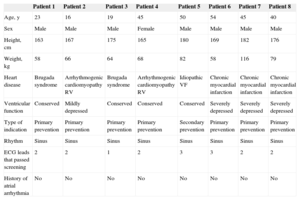
Patient Characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Age, y | 23 | 16 | 19 | 45 | 50 | 54 | 45 | 40 |
| Sex | Male | Male | Male | Female | Male | Male | Male | Male |
| Height, cm | 163 | 167 | 175 | 165 | 180 | 169 | 182 | 176 |
| Weight, kg | 58 | 66 | 64 | 68 | 82 | 58 | 116 | 79 |
| Heart disease | Brugada syndrome | Arrhythmogenic cardiomyopathy RV | Brugada syndrome | Arrhythmogenic cardiomyopathy RV | Idiopathic VF | Chronic myocardial infarction | Chronic myocardial infarction | Chronic myocardial infarction |
| Ventricular function | Conserved | Mildly depressed | Conserved | Conserved | Conserved | Severely depressed | Severely depressed | Severely depressed |
| Type of indication | Primary prevention | Primary prevention | Primary prevention | Primary prevention | Secondary prevention | Primary prevention | Primary prevention | Primary prevention |
| Rhythm | Sinus | Sinus | Sinus | Sinus | Sinus | Sinus | Sinus | Sinus |
| ECG leads that passed screening | 2 | 2 | 1 | 2 | 3 | 3 | 2 | 2 |
| History of atrial arrhythmia | No | No | No | No | No | No | No | No |
ECG, electrocardiogram; VF, ventricular fibrillation; RV, right ventricle.
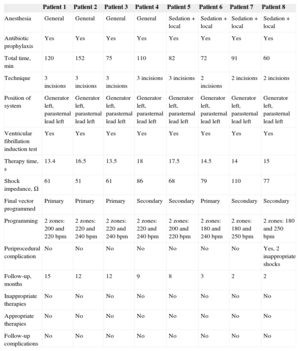
Data Related to Device Implantation and Follow-up
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Anesthesia | General | General | General | General | Sedation + local | Sedation + local | Sedation + local | Sedation + local |
| Antibiotic prophylaxis | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total time, min | 120 | 152 | 75 | 110 | 82 | 72 | 91 | 60 |
| Technique | 3 incisions | 3 incisions | 3 incisions | 3 incisions | 3 incisions | 2 incisions | 2 incisions | 2 incisions |
| Position of system | Generator left, parasternal lead left | Generator left, parasternal lead left | Generator left, parasternal lead left | Generator left, parasternal lead left | Generator left, parasternal lead left | Generator left, parasternal lead left | Generator left, parasternal lead left | Generator left, parasternal lead left |
| Ventricular fibrillation induction test | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Therapy time, s | 13.4 | 16.5 | 13.5 | 18 | 17.5 | 14.5 | 14 | 15 |
| Shock impedance, Ω | 61 | 51 | 61 | 86 | 68 | 79 | 110 | 77 |
| Final vector programmed | Primary | Primary | Primary | Secondary | Secondary | Primary | Secondary | Secondary |
| Programming | 2 zones: 200 and 220 bpm | 2 zones: 220 and 240 bpm | 2 zones: 220 and 240 bpm | 2 zones: 220 and 240 bpm | 2 zones: 200 and 220 bpm | 2 zones: 180 and 240 bpm | 2 zones: 180 and 250 bpm | 2 zones: 180 and 250 bpm |
| Periprocedural complication | No | No | No | No | No | No | No | Yes, 2 inappropriate shocks |
| Follow-up, months | 15 | 12 | 12 | 9 | 8 | 3 | 2 | 2 |
| Inappropriate therapies | No | No | No | No | No | No | No | No |
| Appropriate therapies | No | No | No | No | No | No | No | No |
| Follow-up complications | No | No | No | No | No | No | No | No |
Subcutaneous ICD implantation was performed by 2 electrophysiologists (operator and assistant), with broad experience in implantation of conventional devices. In the first case alone, the procedure was performed under the supervision of an operator experienced in implantation of subcutaneous devices. Fluoroscopy was not used in any procedure. The main data related to the implantation procedure and follow-up are shown in Table 2. We used the previously described implantation technique involving 3 incisions1 in all patients except the last 3, who underwent the new simplified technique of only 2 incisions.3 There were no incidents during the procedure, with the exception of patient 8 who initiated atrial fibrillation after the device had properly detected and treated induced ventricular fibrillation. The device was programmed with 2 zones (180 and 250 bpm) and several minutes later, the patient received 2 inappropriate shocks due to very fast atrial fibrillation (above 180 bpm) with intervals of aberrant conduction. In the induction test, ventricular fibrillation was induced in all patients, with good detection by the device. Adequate defibrillation was achieved with the first programmed 65-J shock in 7 patients; patient 7 needed a second 65-J shock with reversed polarity. The mean duration of therapy was 15.3seconds, with a mean shock impedance of 74.12Ω. In all patients, the device was programmed with 2 antitachycardia zones, 1 conditional with active discrimination algorithms, and 2 in which shocks were delivered exclusively according to the heart rate (Table 2). All patients were discharged from hospital the day after the procedure, following radiologic confirmation of proper positioning of the system. At follow-up, there were no complications or device-related events, lead detection and impedance were adequate, the patients were asymptomatic, and surgical wounds were in perfect condition.
Data from the main multicenter studies using the device show low implant-related complication rates and good performance for detecting, discriminating, and terminating malignant ventricular arrhythmias.4,5 The accumulated experience has enabled optimization of the most suitable mode for programming, which considerably reduced the incidence of inappropriate shocks in the EFFORTLESS study,5 the most recent at the time of writing. Because the device does not provide permanent stimulation, it is not appropriate for patients requiring this capability. It is, however, an attractive option for patients with an indication for primary prevention, patients lacking vascular accesses or with difficult accesses, those at a high risk of infection, and young patients with several types of heart diseases, in whom problems with conventional leads are anticipated because of the length of time they would need an ICD.
Although the initial experience described is positive, it is very limited. The indications, benefits, and real potential of subcutaneous ICDs will have to be determined over the next few years through the results of ongoing multicenter studies6 and implementation of further technical improvements.
CONFLICT OF INTERESTSM.A. Arias is implant proctor in other centers.