Left dominant arrhythmogenic cardiomyopathy (LDAC) has recently been recognized as falling on the spectrum of arrhythmogenic cardiomyopathy. It is characterized by fibroadipose replacement of the left ventricle. The aim of this study was to describe the most frequent forms of clinical presentation of LDAC, imaging findings, and events at follow-up, highlighting the importance of cardiac magnetic resonance (CMR).
MethodsProspective registry of patients with findings compatible with LDAC. CMR image analysis and clinical follow-up was performed. The primary endpoint was the appearance of major adverse cardiovascular events (MACE) during follow-up, defined as sudden cardiac death, sustained ventricular arrhythmias, and heart transplant.
ResultsWe included 74 consecutive patients (mean age, 48.6 years; 50 men [67.6%]). The most frequent CMR indications were chest pain with normal coronary angiography, ventricular arrhythmias, and suspicion of cardiomyopathies. The main CMR findings were midwall and/or subepicardial pattern of late gadolinium enhancement (91.9%), fatty epicardial infiltration (83.8%), and left ventricle segmental contractility abnormalities (47.9%). At a mean follow-up of 3.74 years, 24 patients (32.4%) had a MACE (sudden cardiac death 8.1%, sustained ventricular arrhythmias 21.6%, and heart transplant 4.1%). Independent predictors for the appearance for MACE were a CMR study showing severe late gadolinium enhancement, male sex, and practicing sports.
ConclusionsCMR is a key tool for diagnosing LDAC. Characteristic findings are subepicardial fatty infiltration and midwall-subepicardial late gadolinium enhancement. The prognosis of this population is poor with a high incidence of sudden cardiac death and ventricular arrhythmias.
Keywords
Arrhythmogenic right ventricular cardiomyopathy is a hereditary disease characterized by progressive replacement of the myocardium by adipose and fibrous tissue, predisposing people to ventricular tachycardia and sudden cardiac death (SCD). Fontaine et al.1 were the first to describe arrhythmogenic right ventricular cardiomyopathy in 1977, and a preliminary description of its clinical profile followed 3 years later.2 These pioneer studies paved the way for others, which helped establish arrhythmogenic right ventricular cardiomyopathy as a cardiomyopathy mainly affecting the right ventricle. The involvement of the left ventricle (LV) was considered only as a late manifestation, and only recently have 3 patterns of the disease been recognized: the classic subtype, with its well-known predilection for the right ventricle; the biventricular variant, defined by parallel involvement of both ventricles; and left dominant arrhythmogenic cardiomyopathy (LDAC), characterized by the early and predominant involvement of the LV. The 3 categories show similar age distributions, lending support to the premise that the patterns are independent and coexist in the arrhythmogenic right ventricular cardiomyopathy population.3 The disease is, therefore, increasingly being referred to as arrhythmogenic cardiomyopathy (AC).2,4
LDAC is characterized pathologically by fibroadipose replacement of the LV, often occurring as a circumferential band in the outer third of the myocardium and on the right side of the interventricular septum.5 This fibrofatty infiltration predisposes patients to LV dysfunction and life-threatening ventricular arrhythmias. There are few data in the literature about this entity, with only isolated reports4,6–12 since Sen-Chowdhry et al.5 first established the clinical diagnostic features of LDAC in 2008. These include unexplained T-wave inversion in V5, V6±V4, I, and aVL on electrocardiogram (ECG); arrhythmias such as sustained or nonsustained ventricular tachycardia of right bundle branch block configuration, documented on ECG or Holter monitoring or during exercise testing; frequent ventricular extrasystoles (right bundle branch block morphology); LV aneurysms on imaging study, mild LV dilation and/or systolic impairment (with arrhythmic presentation); biopsy-proven myocyte loss with fibrofatty replacement; and cardiac magnetic resonance (CMR) imaging that shows LV late gadolinium enhancement (LGE) (with subepicardial/midwall myocardial distribution).
Although to date the reported prevalence of LDAC is low, this may be due to under-recognition. The aim of this study was to describe the most frequent forms of clinical presentation of LDAC, imaging findings and events at follow-up, highlighting the importance of CMR.
METHODSPatients and study designWe included prospectively recruited patients with findings compatible with LDAC according to the criteria of Sen-Chowdhry et al.,5 from 2014 to 2018. All CMR studies performed between 2010 and 2014 were also retrospectively reviewed (finally 51 patients were included prospectively and 23 patients retrospectively with CMR compatible findings, that likewise fulfilled the above-mentioned criteria). Family studies conducted in these patients identified relatives with positive genetic tests. Those who had either CMR findings compatible with LDAC or ECG alterations were also included. Patients with coronary disease (ruling out inducible ischemia or a previous history of myocardial infarction) were excluded.
Clinical, personal, and family history were recorded. Patients were referred to a specific cardiomyopathy unit, where they underwent an echocardiogram and 24-hour Holter monitoring. An exhaustive review was performed of follow-up medical data. Clinical records of patients with hospital readmissions and/or outpatient clinic interviews were also reviewed for further information, to validate data in our registry database. We also analyzed arrhythmic events in patients fitted with an implantable cardioverter-defibrillator.
The study protocol was approved by the local ethics committee, and all patients gave written informed consent after being informed of the purpose of this study.
Cardiac magnetic resonanceImage acquisitionA 1.5 T scanner (Intera CV, Philips Medical Systems, Best, the Netherlands) and a 5-element phased-array surface coil were used for all CMR studies. The patients were continuously monitored during the examination with a single-lead ECG and pulse oximetry. Patients were positioned supine, headfirst. All images were acquired with ECG gating and during suspended respiration. Morphologic images in the cardiac short-axis, 4-chamber long-axis, 2-chamber long-axis, and 3-chamber planes were acquired using a standard, steady-state free precession technique. LGE imaging was performed 10minutes after peripheral bolus injection of 0.1 mmol/kg of gadobutrol (Gadovist, Bayer Schering Pharma, Berlin, Germany) using a 3-dimensional inversion recovery turbo gradient echo sequence, again covering the entire myocardium in the short-axis, 4-chamber long-axis, and 2-chamber long-axis planes. Inversion time was individually determined to null normal myocardial signal. In cases in which myocardial thinning was apparent on short-axis cine images, T1 fat-saturated and T1 nonfat-saturated images were also acquired on that plane.
Image analysisAn expert CMR radiologist performed the image analysis on an independent workstation provided by the manufacturer (View-Forum release 6.3, Philips Medical Systems). For ventricular volume analysis, the endocardial border was determined in end-systole and end-diastole for all short-axis images. LV end-diastolic and -systolic volumes, stroke volume, ejection fraction and cardiac output were calculated from a stack of sequential short-axis cine loops (8-12 contiguous slices) by semiautomatic segmentation. Volumes were indexed by body surface. Each segment of the LV was carefully examined to determine the presence of either fatty infiltration (visually assessed in cine images, defined as epicardial irregular contour and/or myocardial thinning at the expense of the epicardium), LGE, or both. Data were collected on a circumferential polar plot of the 17 myocardial segments. The pattern of LGE (subendocardial, midwall, subepicardial, or transmural) was also assessed.
Clinical endpointsThe primary clinical endpoint was the appearance of major adverse cardiovascular events (MACE) during follow-up, defined as SCD, sustained ventricular arrhythmias, and heart transplant. The secondary endpoint was the individual study of the variables that formed the composite endpoint as well as the rate of cardiac readmission for heart failure. When the reason for requesting a CMR was the presence of sustained ventricular arrhythmias or aborted SCD, these episodes were considered events after the diagnosis of LDAC.
Statistical analysisContinuous variables were tested for normality using the Kolmogorov-Smirnov test. Normally distributed variables are presented as mean±standard deviation, while nonparametric variables are expressed as median and interquartile range [IQR]. Discrete variables are presented as relative frequencies, which were compared using the chi-square test or Fisher tests, as appropriate. For continuous variables, the Student t test was used.
The variables identified as predictors of MACE on univariable analysis (hazard ratios [HR] <0.3) were included in a multivariable Cox regression with a conditional stepwise forward model (pin <0.10, pout <0.05) to correct for colinearity. Adjusted HR and 95% confidence intervals (95%CI) were computed for variables independently associated with MACE.
All tests were 2-tailed, and a P value <.05 was considered as statistically significant. Analyses were performed with SPSS (version 15.0, SPSS Inc, Chicago, Ill).
RESULTSWe included 74 consecutive patients (mean age, 48.6 years; 50 men [67.6%]). Hypertension was present in 20.3% of the patients, and diabetes in 6.8%. ECG changes (T-wave inversion in I, aVL, II, III, aVF leads) were present in 47.8% of the patients. Baseline characteristics are shown in table 1.
Baseline population characteristics (N=74)
| Variable | Participants |
|---|---|
| Age at diagnosis, y | 48.6±15.9 |
| Men | 50 (67.6) |
| Weight, kg | 79.8±18.2 |
| Height, cm | 170.9±10.8 |
| Body surface index, m2 | 1.9±0.2 |
| Hypertension | 15 (20.3) |
| Diabetes | 5 (6.8) |
| Family history of SCD of unknown cause | 19 (31.1) |
| T-wave inversion I, aVL, II, III, aVF | 33 (47.8) |
| Documented ventricular arrhythmia of LV or biventricular origin | 49 (66.2) |
| Relatives with diagnosed LDAC | 20 (28.2) |
LDAC, left dominant arrhythmogenic cardiomyopathy; LV, left ventricular; SCD, sudden cardiac death.
The data are expressed as No. (%) or mean±standard deviation.
Indications for CMR studies are described in table 2. The most frequent indications in symptomatic patients were chest pain with normal coronary angiography, ventricular arrhythmias, and suspicion of cardiomyopathies in the echocardiographic study. The largest percentage was the group of patients referred for family screening of AC (31.5%).
Indications for CMR study and CMR finding (N=74)
| Indications for CMR study | |
| Chest pain with normal coronary angiography or suspected myocarditis | 16 (21.6) |
| Frequent ventricular extrasystole | 5 (6.7) |
| Sustained ventricular arrhythmias | 11 (14.9) |
| Aborted sudden cardiac death | 3 (4.1) |
| Cardiomyopathies | 16 (21.6) |
| Dilated cardiomyopathy | 14 (18.9) |
| Hypertrophic cardiomyopathy | 2 (2.7) |
| Relatives with sudden cardiac death or arrhythmogenic cardiomyopathy | 23 (31.1) |
| CMR findings | |
| Midwall and/or subepicardial pattern of LGE | 68 (91.9) |
| Mean number of segments with fibrosis | 10.1±5.6 |
| Location of fibrosis | |
| Septum | 42 (56.8) |
| Inferior wall | 61 (82.4) |
| Inferolateral wall | 64 (86.5) |
| Anterolateral wall | 61 (82.4) |
| Anterior wall | 47 (63.5) |
| Fatty epicardial infiltration | 62 (83.8) |
| Location of fatty infiltration | |
| Septum | 23 (31.1) |
| Inferior wall | 52 (70.3) |
| Inferolateral wall | 60 (81.1) |
| Anterolateral wall | 58 (78.4) |
| Anterior wall | 39 (52.7) |
| LV segmental contractility abnormalities | 34 (47.9) |
| Mean LVEDV index, mL/m2 | 95.0±20 |
| Mean LVESV index, mL/m2 | 51.6±26.3 |
| Mean LVEF, % | 47.6±11.8 |
| RV dilatation | 12 (16.2) |
| RV systolic dysfunction, RVEF <50% | 16 (21.6) |
CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; LV, left ventricle; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LVEF, left ventricular ejection fraction; RV, right ventricle; RVEF, right ventricular ejection fraction.
The data are expressed as No. (%) or mean±standard deviation.
The most frequent morphological CMR findings were midwall and/or subepicardial pattern of LGE (91.9%), fatty epicardial infiltration (83.8%), and LV segmental contractility abnormalities (47.9%) (table 2). Mean left ventricular ejection fraction was 47.6% (standard deviation 11.8) and 47.3% were dilated (indexed by body surface). In almost half of the cases, the previous echocardiogram was normal. RV systolic dysfunction (right ventricular ejection fraction <50%) was present in 16 patients (21.6%).
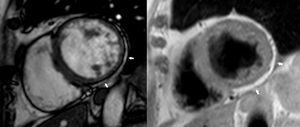
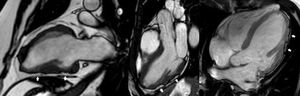
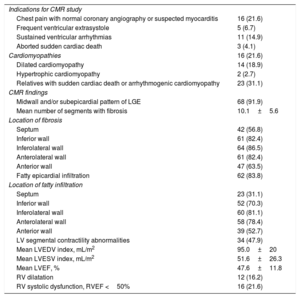
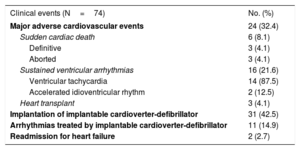
The most frequent location of the fibrosis was the inferior and inferolateral walls (n=64), while the involvement of the septum was less frequent. The amount of LV fibrosis was strongly related to the amount of fatty infiltration. In fact, they both had a similar distribution. Fatty infiltration was observed differently depending on the acquisition plane: in short-axis cine views, it was depicted as a circumferential subepicardial band of fatty tissue, usually on the inferior and lateral walls (further demonstrated with T1-weighted images) (figure 1); in 2-, 3- and 4-chamber cine images, it appeared as focal epicardial contour irregularities, which we called “the rat-bite sign” after the resemblance to a piece of cheese bitten by this animal (figure 2).
Patients with more LV segments affected by fatty infiltration and fibrosis were more likely to have depressed LV function and higher LV volumes.
Follow-upAll patients were followed up for a mean of 3.74 years. Twenty-four patients (32.4%) had 1 or more major event during this period: 16, sustained ventricular arrhythmia (21.6%); 3, aborted SCD (4.1%); 3 definitive SCD (4.1%); and 3, heart transplants (4.1%) (table 3).
Long-term clinical outcomes
| Clinical events (N=74) | No. (%) |
|---|---|
| Major adverse cardiovascular events | 24 (32.4) |
| Sudden cardiac death | 6 (8.1) |
| Definitive | 3 (4.1) |
| Aborted | 3 (4.1) |
| Sustained ventricular arrhythmias | 16 (21.6) |
| Ventricular tachycardia | 14 (87.5) |
| Accelerated idioventricular rhythm | 2 (12.5) |
| Heart transplant | 3 (4.1) |
| Implantation of implantable cardioverter-defibrillator | 31 (42.5) |
| Arrhythmias treated by implantable cardioverter-defibrillator | 11 (14.9) |
| Readmission for heart failure | 2 (2.7) |
In 31 patients (42.5%), implantable cardioverter-defibrillator implantation was indicated (14 patients due to sustained ventricular tachycardia, 6 due to decreased left ventricular ejection fraction, 7 due to extensive fibrosis and positive genetics for LDAC, 2 due to syncope and 2 due to aborted SCD). In 1 patient with aborted SCD, no implantable cardioverter-defibrillator was placed due to severe postanoxic encephalopathy). During follow-up, appropriate therapies were registered mostly due to ventricular tachycardia (n=11, 14.9%): 9 patients had sustained ventricular tachycardia, 1 ventricular fibrillation, and 1 both sustained ventricular tachycardia and ventricular fibrillation.
Features related to the presence of events during follow-up are shown in table 4. Male sex, sports practice (3 out of 6 SCDs in our population occurred during sporting activities), and ECG changes were significantly associated with the incidence of MACE. In terms of CMR findings, the presence of LGE alone did not predict major events, but there was an increased tendency to more events in patients with a higher number of LV segments affected by fibrosis, especially when more than 15 segments were involved. The presence of fatty infiltration was not related to the events.
Associations between different characteristics and events at follow-up
| Variables | MACE | No MACE | P |
|---|---|---|---|
| Age, y | 48.5±17.0 | 48.6±15.5 | .970 |
| Male sex | 20 (87.0) | 30 (58.8) | .018 |
| Hypertension | 5 (21.7) | 10 (19.6) | .830 |
| Diabetes | 3 (13.0) | 2 (3.9) | .150 |
| Sport practice | 8 (34.8) | 1 (2.0) | .001 |
| LGE | 23 (100) | 45 (88.2) | .170 |
| Severe LGE (> 15 affected segments) | 11 (47.8) | 12 (24.0) | .058 |
| Fatty infiltration | 22 (95.7) | 40 (78.4) | .065 |
| Inferolateral wall fatty infiltration | 22 (95.7) | 36 (70.6) | .016 |
| T-wave inversion I, aVL, II, III, aVF | 13 (68.4) | 20 (40.0) | .058 |
| LVEDV index, mL/m2 | 108.8±38.1 | 88.9±23.1 | .008 |
| LVESV index,mL/m2 | 65.0±34.0 | 45.3±19.4 | .003 |
| LVEF, % | 41.6±11.8 | 50.6±10.5 | .002 |
| RVEF, % | 51.2±13.1 | 54.2±9.6 | .349 |
| Positive genetic test | 9 (39.1) | 37 (57.1) | .078 |
LGE, late gadolinium enhancement; LVEDV, left ventricle end diastolic volume; LVEF, left ventricle ejection fraction; LVESV, left ventricle end systolic volume; MACE, major adverse cardiovascular events; RVEF, right ventricular ejection fraction.
Data are expressed as No. (%) or mean±standard deviation.
The predictors for MACE are shown in table 5. Multivariable analysis demonstrated that independent variables associated with MACE were male sex (HR, 7.99; 95%CI, 1.55-41.32; P=.013), severe LGE (> 15 segments affected) (HR, 3.77; 95%CI, 1.23-11.44; P=.019), and sports practice (HR, 6.60; 95%CI, 2.08-20.94; P=.001).
Independent predictors of MACE. Cox regression analysis
| Variable | HR | 95%CI | P |
|---|---|---|---|
| Male sex | 7.99 | 1.55-41.32 | .013 |
| T-wave inversion I, aVL, II, III, aVF | 2.06 | 0.28-15.25 | .481 |
| Dilatation LV | 0.40 | 0.10-1.57 | .189 |
| LV segmental contractility abnormalities | 3.09 | 0.36-26.95 | .306 |
| Severe LGE (> 15 segments affected) | 3.77 | 1.23-11.44 | .019 |
| Presence of fatty infiltration | 0.61 | 0.06-6.47 | .682 |
| Sports practice | 6.60 | 2.08-20.94 | .001 |
95%CI, 95% confidence interval; HR, hazard ratio; LGE, late gadolinium enhancement; LV, left ventricle; MACE, major adverse cardiovascular events.
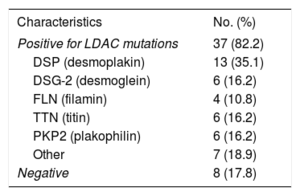
Autopsy performed in 1 case of SCD was positive for LDAC. At the time of writing, we had received the results of 45 genetic tests (60.8%): 8 were negative and 37 positives (82.2%). In the study of the probands, the diagnostic performance of the genetic test was 72%. Mutations included those on the filamin (FLN), desmoglein (DSG2), desmoplakin (DSP), and titin (TTN) genes (table 6). Some patients were positive for more than 1 mutation. The analysis performed according to the result of the genetic test did not show any differences in terms of left ventricular ejection fraction, degree of fibrosis, or the development of events, and it was not an independent predictor of MACE.
Genetic test results (N=45)
| Characteristics | No. (%) |
|---|---|
| Positive for LDAC mutations | 37 (82.2) |
| DSP (desmoplakin) | 13 (35.1) |
| DSG-2 (desmoglein) | 6 (16.2) |
| FLN (filamin) | 4 (10.8) |
| TTN (titin) | 6 (16.2) |
| PKP2 (plakophilin) | 6 (16.2) |
| Other | 7 (18.9) |
| Negative | 8 (17.8) |
LDAC, left dominant arrhythmogenic cardiomyopathy.
To date, this is the largest published series of patients with LDAC diagnosed by CMR. In our experience, CMR is an invaluable tool for diagnosing this entity. The main results of our study are as follows. First, subepicardial fatty infiltration (better displayed on 2-, 3- and 4 chamber-view cine images as irregularities on the epicardial contour, in a pattern we call “the rat-bite sign”) was present in 83.8% of cases. Secondly, midwall-subepicardial LGE was observed in 91.9% of patients, usually affecting the inferior and lateral walls and frequently extending to the anterior and septal walls. Third, the prognosis of this population was poor, with a high incidence of SCD and ventricular arrhythmias. Finally, there was a high proportion of genetic tests that were positive for LDAC (72% of the probands).
In 1994, the Task Force criteria were established for arrhythmogenic right ventricular cardiomyopathy.13 These were modified in 2010 and included numerous structural, histopathological, electrocardiographic, familial, arrhythmic, and genetic parameters.14 However, these criteria were derived from cohorts with right ventricular forms of the disease, since biventricular or left dominant patterns had not yet been contemplated. This means that the criteria could potentially be inadequate for a significant proportion of patients whose disease followed these other patterns.
In 2008, Sen-Chowdhry et al.5 established the clinical diagnostic features of LDAC. Since then, only a few reports describing isolated cases4,6–11 or certain genetic mutations associated with the entity12,15–17 have been published.
In the retrospective series of patients with sudden death due to AC by Miles et al.,18 70% of the patients showed biventricular involvement at autopsy, and none of these would have met the Task Force criteria. Therefore, left ventricular variants of arrhythmogenic cardiomyopathy may evade clinical detection using current diagnostic tools. We believe this should be addressed in future revisions of the Task Force criteria. Furthermore, in this same series, over half of the decedents diagnosed with cardiomyopathy in life were labelled as having dilated cardiomyopathy but showed pathological features of AC at autopsy. The overlap between dilated cardiomyopathy and AC may present several diagnostic challenges, especially if the only available imaging technique is echocardiography. Thus, CMR is of the utmost importance in the diagnosis of this entity, enabling the detection of fatty infiltration and fibrosis.
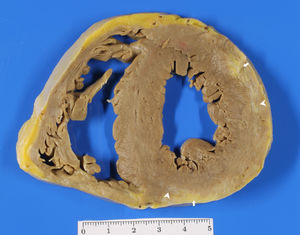
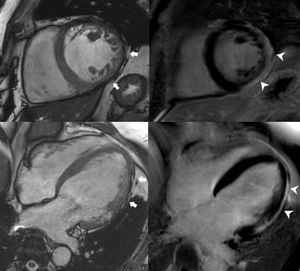
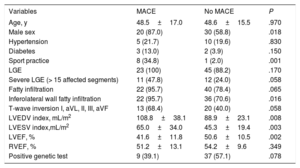
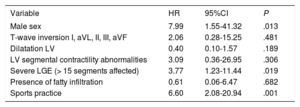
In our series, fatty infiltration was present in 83.8% and late enhancement in 91.9% of our patients. Fibrosis, which has a greater presence on the inferolateral wall, could indicate the onset of disease in these myocardial segments. We identify a new sign, named “the rat-bite,” which has not yet been described in the literature. The characteristic subepicardial fatty infiltration found in macroscopic samples of patients with LDAC (figure 3) is very well depicted on cine-MR images as focal epicardial contour irregularities that resemble bitten cheese. This feature is always associated with LGE (figure 4), representing the fibrofatty infiltration, and it is highly specific to this entity. This sign can be highly useful for diagnosing patients with LDAC. Patients with the biventricular pattern of the disease show similar signs.
Short-axis still cine image (upper left) showing myocardial thinning on the inferior and lateral walls (arrows) and corresponding LGE image (upper right) in which subepicardial fibrosis can be demonstrated on those locations (arrowheads). Likewise, on the lower images, epicardial contour irregularities on the lateral wall can be demonstrated (arrows) on the 4-chamber still cine image (lower left) and fibrosis in the same location (arrowheads) on the LGE image (lower right). LGE, late gadolinium enhancement.
The clinical presentation of LDAC is heterogeneous, and its diagnosis can be challenging. In our series, the main indications for a CMR study were sustained ventricular arrhythmias, chest pain with normal coronary angiography or suspected myocarditis, and dilated cardiomyopathy. About 10 years ago, we were satisfied with signs such as chest pain with normal coronary arteries and mild troponin elevation. If there was a minimal history of infection (eg, gastroenteritis, a cold), patients were diagnosed with myocarditis, and no further analysis or follow-up were indicated. Patients with a normal echocardiogram who were otherwise asymptomatic were discharged. Recently, myocardial infarction with no obstructive coronary atherosclerosis (MINOCA) guidelines have shed some light on the approach to patients with chest pain, troponin elevation and a normal coronary angiogram.19 Nowadays, in our area (which includes 1 tertiary hospital and 5 secondary hospitals), patients with chest pain and normal coronary arteries usually undergo a CMR study, either during admission or shortly after discharge. Many patients receive a final diagnosis of infarction with normal coronary arteries, myocarditis or, less frequently (but no less importantly) AC.
We consider showing the prognostic impact of this disease to be of utmost importance. Arrhythmogenic right ventricular cardiomyopathy has a poor prognosis in the mid-term, with a high incidence of major events,20 but the prognosis of LDAC was heretofore unknown. At a mean follow-up of 3.74 years, 24 of our patients (32.4%) had a MACE, including SCD in 8.1%, sustained ventricular arrhythmias in 21.6%, and heart transplant in 4.1%.
Thus, a novel contribution of this report is a list of prognostic predictors that could be very useful in the clinical follow-up of these patients. A CMR study showing severe LGE (> 15 affected segments) (aHR=3.77), independently predicts the appearance of MACE, as well as other clinical factors such male sex (aHR=7.99), and sports practice (aHR=6.60).
Another aspect to highlight is the relevance of the genetic study. In our series, 72% of the genetic studies performed in probands were positive for mutations related to this entity, helping to confirm the suspected diagnosis and to establish the basis for family screening. Relatives with positive mutations may undergo a stricter follow-up and receive behavioral advice, for example to avoid sport, even in the absence of structural findings, with the aim of slowing down disease development.
The natural history of LV involvement in AC is poorly understood. There have been series showing no association between LV involvement and age at death. In addition, the macroscopic appearance of the heart at autopsy corroborates imaging studies that suggest that LV involvement may occur at an early stage.18 Therefore, we should be cautious with the relatives of patients with AC who have negative image tests but positive gene mutations. In our series, there were 2 such relatives of patients with AC, whose young age could explain the absence of structural findings. For these patients, we consider follow-up mandatory to monitor their progress and, if needed, offer them appropriate therapy.
Another issue we find crucial is direct communication between the CMR expert and the clinician, going beyond isolated consultation on image findings. Other diagnostic criteria must be investigated further and genetic tests requested when needed. Synergy between the physician who analyzes the images and the attending clinician who treats the patient is necessary to enable early intervention and correct screening of family members. This symbiosis, grounded in a good understanding of both radiological findings and other clinical parameters (family history, personal history, clinical, follow-up), also favors the avoidance of potentially erroneous oversensing.
LimitationsAC with predominant involvement of the LV is a less prevalent and less understood entity,9,21 with no defined diagnostic criteria, so there are no clinical trials or specific meta-analyses of this disease. Because our hospital is a reference center in CMR studies for several specialized cardiomyopathy units, our series is relatively large. In addition, a single, experienced radiologist sees all the cases, so there is no interobserver variability. However, in absolute terms the number is still small, precluding us from drawing definitive conclusions.
It should be noted that the ambispective nature of this study can increase the diagnostic performance of CMR by including retrospective patients with findings compatible with the disease studied.
Another limitation could be that genetic testing was not performed in all patients, due to a decision of the referring clinicians, nor was screening performed in all relatives. This could entail a risk of information bias, and relatives could go undiagnosed.
Finally, as discussed above, some relatives may be screened with imaging tests (mainly CMR) at very early ages. In these patients, a negative result does not necessarily rule out disease that could be too premature to produce structural image findings.
Even so, we believe that our series highlights the value of thinking about this disease, being alert to the findings of CMR studies, and performing appropriate family screening in order to guide these patients in the best possible way, both diagnostically and therapeutically.
CONCLUSIONsCMR is a key tool for diagnosing LDAC: subepicardial fatty infiltration is present in about 83.8% of patients, and midwall-subepicardial LGE in about 91.9%, usually affecting the inferior and lateral walls. The prognosis of this population is poor with a high incidence of SCD and ventricular arrhythmias. The presence of a positive genetic test in patients with LDAC diagnosed by CMR is very high (72%).
One contribution of this study is the identification of a characteristic image finding—irregular epicardial contour seen on CMR cine images, denominated here as “the rat-bite sign”—which we describe for the first time. Revisions of AC diagnostic criteria could consider this as one of the included image features.
- -
LDAC is 1 of 3 different patterns of presentation of arrhythmogenic cardiomyopathy. It consists of subepicardial fibrofatty replacement of the LV wall, predisposing patients to LV impairment, arrhythmias and, possibly, SCD. Some clinical features have already been described but we do not know the main findings present in CMR studies.
- -
The absence of patient records with this disease makes us unaware of the prognosis of this entity as well as the existence of possible event predictors.
- -
This is the largest series of patients with LDAC diagnosed by CMR. CMR is an invaluable tool for diagnosing this entity.
- -
The main CMR findings were the presence of subepicardial fatty infiltration (83.8%) and midwall-subepicardial LGE (91.9%). We also contribute a new CMR feature, not previously described, that we consider highly characteristic of this entity: the rat-bite sign, which is the image representation of the subepicardial LV wall fatty infiltration.
- -
We also highlight the importance of CMR in the diagnosis of this disease, the diverse forms of clinical presentation, predictors of events, and its poor prognosis.
None declared.