About 5% to 10% of patients referred for catheterization with an initial diagnosis of myocardial infarction have no obstructive lesions on coronary angiography, an entity known as myocardial infarction with nonobstructive coronary arteries (MINOCA). Interest in this condition has grown in recent years, leading to a position paper1 and specific management recommendations in the European guidelines on non–ST-segment elevation acute coronary syndrome.2 In an observational study recently published by García-Blas et al. in Revista Española de Cardiología,3 the authors analyzed the long-term prognosis of patients with MINOCA, with special focus on the impact of the atherosclerotic plaque burden. The study included 591 consecutive patients with infarction; 20% had no obstructive lesions. The results showed that the 5-year risk of major adverse cardiovascular events (MACE) was lower in patients with MINOCA than in those with obstructive disease, with a relative risk of 0.63. One of the most valuable aspects of the work is that the authors analyzed the prognosis of the 121 patients with MINOCA by plaque burden—smooth coronary arteries, mild disease (0%-30% stenosis), and moderate disease (30%-49%)—and found a major increase in MACE risk with each level of atheromatosis. Using patients with smooth coronary arteries as the reference group, the relative risks of mild and moderate disease were 2.45 and 3.64, respectively. Thus, it appears that even nonsignificant coronary artery atheromatosis affects the prognosis of patients with MINOCA. Notably, these outcomes are clearly worse than those of patients with chronic coronary syndromes: the event rate in patients with MINOCA and moderate disease was > 50% at 5 years, which is 3 times higher than that found in the registry cohort of the FAME 2 trial.4 This finding highlights the potentially unstable nature of coronary heart disease in MINOCA and underlies the importance of a good pathophysiological diagnosis in these patients (figure 1).
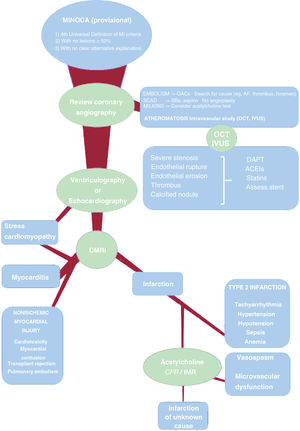
Proposed diagnostic stratification of MINOCA. AMI, acute myocardial infarction; BBs, beta-blockers; CFR, coronary flow reserve; CMRI, cardiac magnetic resonance imaging; DAPT, dual antiplatelet therapy; IMR, index of microcirculatory resistance; IVUS, intravascular ultrasound; MINOCA, myocardial infarction with nonobstructive coronary arteries; OACs, oral anticoagulants; OCT, optical coherence tomography; SCAD, spontaneous coronary artery dissection.
The frequency and prognosis of MINOCA vary among studies, largely due to the considerable terminological confusion and methodological variabilities. Both the European position paper1 and the European guidelines on non–ST-segment elevation acute coronary syndrome2 have attempted to standardize the terminology and guide the diagnostic and therapeutic process for MINOCA. Nonetheless, the wide range of diseases covered by this term, as well as the multitude of recommended diagnostic studies and lack of a standardized protocol, means that the diagnostic and therapeutic strategies in these patients are highly variable and not always based on the best evidence. Understandably, many clinicians and interventional cardiologists believe that the type of exhaustive study that appears to be proposed in the guidelines for all patients with MINOCA is not realistic in Spain (for organizational and economic reasons). Here, we advance a proposal to address this problem in a practical and realistic manner in our setting.
Operationally, the term MINOCA must be understood as a provisional working diagnosis. Thus, patients classified as having MINOCA because they meet the criteria of the Universal Definition of Myocardial Infarction do not have significant lesions (50%) on angiography and currently have no clear alternative diagnosis (eg, pulmonary embolism or myocarditis).1 It must be stressed that what these patients definitely have is myocardial injury, but not necessarily infarction, which has an ischemic origin by definition. From there, the diagnostic process must begin with the aim of identifying the specific etiological mechanism (eg, myocarditis, coronary embolism, and infarction due to unstable atherothrombotic plaque). This will lead to a definitive diagnosis and, if possible, a targeted therapy. In the ideal situation, no patient would be discharged with a diagnosis of MINOCA but with a more specific diagnosis. In practice, even after a systematic study, a minority of patients will lack an etiological diagnosis, but at least we will have established or excluded an ischemic mechanism and ruled out the most important causes of nonischemic myocardial injury.
Given that the provisional diagnosis of MINOCA is established via coronary angiography, the first and often most important step in a more in-depth study begins while the patient is still in the catheterization laboratory. A detailed examination of the images can reveal a cause of the infarction in the epicardial arteries, without additional costs or risks for the patient. Thus, abrupt distal blockage of a small vessel indicates a coronary embolism; imaging findings of a double lumen, radiolucency, or diffuse moderate narrowing indicates spontaneous coronary artery dissection; an intramyocardial trajectory of the anterior descending artery is frequently associated with endothelial dysfunction and ischemia caused by vasospasm; and, finally, the presence of nonsignificant atheromatosis should indicate the possible presence of a complicated plaque, often with endothelial rupture and associated thrombus.
The latter case is very important because, as indicated in the article by García-Blas et al.,3 the presence of nonsignificant plaques is a prognostic marker, probably largely due to their instability and vulnerability. Because angiography has low accuracy for determining plaque stability, plaques should be studied with intravascular ultrasound (IVUS) and optical coherence tomography (OCT). Due to its improved spatial resolution, the latter modality enables better assessment of the presence of markers of plaque instability or vulnerability: endothelial rupture, thrombus, erosion, thin-cap fibroatheroma, or calcified nodule. In addition, imaging often elucidates that a plaque appearing to be mild on angiography is actually obstructive.5 IVUS in patients with MINOCA has revealed that the lesions corresponding to a plaque with endothelial rupture show higher plaque burden and greater positive remodeling, which illustrates how mild, apparently innocent, stenoses on angiography can hide a large unstable plaque.6 All of this aids the decision-making regarding the treatment of lesions with angioplasty if required or the indication for intensive drug therapy. Unfortunately, intracoronary imaging was infrequently used in the article by García-Blas et al.,3 which reflects the reality of the clinical practice in many centers. This impedes a deep exploration of the specific mechanisms underlying the significantly negative impact of nonsignificant atheromatosis on prognosis. When systematically used in small series, OCT reveals plaque rupture in at least one-third of patients.7–9 If this modality fails to indicate a specific cause and the patient has not previously undergone echocardiography, left ventriculography performed in the catheterization laboratory can support a diagnosis of stress cardiomyopathy or reveal a regional contraction pattern suggesting an ischemic etiology. Diffuse systolic left ventricular dysfunction would suggest cardiomyopathy or myocarditis.
If the detailed invasive assessment fails to reveal a specific cause of the myocardial injury, it must be remembered that one-third of patients with an initial diagnosis of MINOCA have myocarditis.10 Predictors of this etiology are younger age, male sex, and smooth coronary arteries on angiography.10 Accordingly, the next logical step is cardiac magnetic resonance imaging. Magnetic resonance distinguishes among myocardial infarction, myocarditis, and stress cardiomyopathy based on the presence and distribution of delayed enhancement and edema. Thus, a combined strategy of OCT followed by cardiac magnetic resonance imaging has shown high diagnostic yield in some small studies and enabled etiological diagnosis in more than 85% of patients in some series.7–9
If thorough examination of the coronary arteries and magnetic resonance does not yield a definite diagnosis, we must consider a heterogeneous group of causes of type 2 infarction (eg, tachyarrhythmia, anemia, sepsis), of nonischemic myocardial injury (eg, chemotherapy-related cardiotoxicity, transplant rejection), and of extracardiac diseases (eg, pulmonary thromboembolism, cerebral infarction). This is obviously performed in parallel to the above tests and is based on the medical history, physical examination, and basic tests performed in all patients with acute coronary syndrome, and the initial assessment will directly indicate one of these causes in some patients.
Finally, 2 causes mentioned in the European position paper warrant special attention: vasospasm and microvascular dysfunction. Regarding the first, a meta-analysis11 determined a 28% prevalence of positive provocation tests in patients with MINOCA. Nonetheless, the studies included in this analysis were very small and heterogeneous and the prevalences identified varied between 0% and 95%. Thus, better evidence in this regard is clearly required. Regarding microvascular dysfunction, its prevalence is about 30% in patients with angina without coronary artery disease12 and there are no specific data from patients with MINOCA. Accordingly, a considerable percentage of patients with MINOCA would be expected to have microvascular dysfunction. However, this does not necessarily lead to the conclusion that the microvascular dysfunction is the cause of the infarction. Indeed, in cases of thrombotic or embolic infarction, microvascular dysfunction could be a consequence of the infarction. Microvascular dysfunction is also found in most cardiomyopathies. Taken together, vasospasm and microvascular dysfunction should be considered possible causes but an invasive study (acetylcholine test13 and measurement of coronary flow reserve and microvascular resistances) should probably be reserved for selected patients whose previous systematic study failed to yield results (except in cases with high initial clinical suspicion of vasospasm).
The objective of this diagnostic process is to lay the groundwork for a specific treatment. There are few data on the effectiveness of the standard treatment in MINOCA. The best evidence probably comes from a substudy of the SWEDEHEART trial, published a few years ago.14 This analysis, which included almost 10 000 patients with MINOCA, showed a significant reduction in MACE with statins and angiotensin-converting enzyme inhibitors and a tendency for a reduction with beta-blockers but no beneficial effects with dual antiplatelet therapy. The randomized trial MINOCA-BAT,15 which has a factorial design, will study the efficacy of beta-blockers and angiotensin-converting enzyme inhibitors in patients with MINOCA. This trial will add to the scientific evidence on these drugs but will nonetheless include a relatively heterogeneous cohort, given that its design does not specify a systematic diagnostic strategy, which is why it is likely to include patients with myocarditis and stress cardiomyopathy, among other causes.
We believe that individualized management should be the basic principle in these patients. To give just one example, dual antiplatelet therapy failed to obtain a benefit in the SWEDEHEART study, but the finding of a ruptured thrombotic plaque, with or without subsequent stent implantation, would evidently indicate this therapy. In contrast, in a patient with coronary embolism due to atrial fibrillation, the indicated therapy is anticoagulation and the addition of dual antiplatelet therapy would be useless or even harmful.
Scientific validation of individualized management could be obtained via 2 types of studies. The first type of study would evaluate the efficacy of particular treatments in specific situations related to MINOCA, such as the benefit of statins or dual antiplatelet therapy according to the burden or characteristics of the plaque found in IVUS or OCT. The second type would comprise clinical studies assessing the efficacy of a stratified diagnostic and treatment strategy, as performed by the CorMicA trial16 in the context of angina with coronary artery disease. Within this diagnostic strategy for MINOCA, analysis of coronary atheromatosis and the search for high-risk characteristics via intracoronary imaging should play a central role in the coming years.
FundingThis article has not received funding.
Conflicts Of InterestThe authors have no conflicts of interest to declare.