Discordant data have been reported on the prognosis of myocardial infarction with nonobstructive coronary arteries (MINOCA). Moreover, few data are available on the impact of angiographic subtypes. The objectives of this study were to assess the prognostic impact on the long-term follow-up of the diagnosis of MINOCA and its angiographic subtypes.
MethodsWe included 591 consecutive patients with non–ST-segment elevation myocardial infarction (NSTEMI) who underwent coronary angiography. MINOCA was classified according to angiographic findings as smooth coronary arteries, mild irregularities (< 30% stenosis), and moderate atherosclerosis (30%-49% stenosis). The primary endpoint was a composite of mortality, nonfatal myocardial infarction, and revascularization (MACE) at a median of 5 years of follow-up.
ResultsA total of 121 patients (20.5%) showed no obstructive lesions. MINOCA was associated with a lower occurrence of MACE (P=.014; HR, 0.63; 95%CI, 0.44-0.91) and was confirmed as an independent factor in the multivariate analysis (P=.018; HR, 0.63; 95%CI, 0.43-0.92). On analysis of the separate components of the main endpoint, MINOCA was significantly associated with a lower rate of myocardial infarction and revascularization, but not with mortality. Analysis of angiographic subtypes among MINOCA patients showed that smooth coronary arteries were a statistically significant protective factor on both univariate and multivariate analysis, while mild irregularities and 30% to 49% plaques were associated with a higher risk of MACE.
ConclusionsMINOCA is associated with a lower rate of MACE, driven by fewer reinfarctions and revascularizations. Within the angiographic subtypes of MINOCA, smooth arteries were independently associated with a lower number of MACE.
Keywords
Between 5% and 25% of patients presenting with an acute myocardial infarction (MI) do not show significant coronary stenosis on coronary angiography (myocardial infarction with nonobstructive coronary arteries, MINOCA).1–11 Discordant data have been found on the prognosis of patients with MINOCA, which has been described as better,1,4,9,10,12 similar,2,7,8 or even worse2,13 than the finding of obstructive coronary lesions. Most studies provide data at 1 year, some focus on in-hospital events, but long-term follow-up is exceptional. It cannot be considered a “benign” entity, given that the studies comparing the age- and sex-matched general population have confirmed a worse prognosis for MINOCA and that the literature consistently shows a total annual mortality rate between 4% and 5%.2,4
Current diagnostic criteria for MINOCA include a diagnosis of myocardial infarction (MI) according to its universal definition, nonobstructive coronary arteries (ie, no lesions ≥ 50% in a major epicardial vessel), and the absence of alternative cause.14,15 However, the American Heart Association (AHA) has recently recommended categorizing MINOCA into angiographically normal coronary arteries, mild irregularities (< 30% stenosis), and moderate coronary atherosclerosis (30%-49% stenosis).15 Few data are available on the differential impact of these angiographic subtypes on the prognosis of patients with MINOCA.16,17
This study analyzed a cohort of patients with a diagnosis of MINOCA categorized according to the angiographic subtypes. The patients were followed up for 5 years. The objectives were to assess the prognostic impact of a diagnosis of MINOCA and its angiographic subtypes at long-term follow-up.
MethodsThis was a single-center, observational, consecutive cohort study of patients (from 1 November 2010, to 28 February 2014) admitted to hospital for non–ST-segment elevation myocardial infarction (NSTEMI) who underwent coronary angiography. Exclusion criteria were diagnosis of cardiomyopathy, nonischemic myocardial injury (ie, myocarditis, tako-tsubo syndrome) or noncardiac origin of the clinical picture, and previous coronary artery bypass grafting. This project is included within the framework of the “registry of patients admitted to the cardiology ward for chest pain”, which has been approved by the local clinical research ethics committee. Patients were managed at the discretion of the treating physician, following clinical practice guidelines.18
Presentation and admission data were systematically collected through a specific database (table 1, table 2, and table 3). Regarding troponin (Tn) analysis, Elecsys high sensitivity cardiac TnT assay (Roche Diagnostics, Switzerland) was used; 14 ng/L corresponds to the 99th percentile cutoff for a healthy reference population. A first Tn determination was performed at admission and a second one was scheduled within the first 6hours, as recommended in the current clinical guidelines at the beginning of the study period.19 Moreover, peak Tn level during admission and delta Tn (estimated as the absolute or relative change between the first and second Tn measurements) were also analyzed.
Baseline characteristics: previous medical history
| Overall sample (n=591) | Patients with coronary obstructive lesions (n=470) | Patients with nonobstructive coronary arteries (n=121) | P | |
|---|---|---|---|---|
| Male sex | 405 (68.5) | 341 (72.6) | 64 (52.9) | < .001 |
| Age | 68.8±13.0 | 68.9±13.0 | 68.3±13.2 | .61 |
| High blood pressure | 427 (72.3) | 342 (72.8) | 85 (70.2) | .519 |
| Dyslipidemia | 346 (58.5) | 279 (59.4) | 67 (55.4) | .281 |
| Diabetes | 215 (36.4) | 181 (38.5) | 34 (28.1) | .016 |
| Smoker | 143 (24.2) | 124 (26.4) | 19 (15.7) | .013 |
| Family history | 27 (4.6) | 24 (5.1) | 3 (2.5) | .316 |
| Any cardiovascular risk factor | 550 (93.1) | 443 (94.3) | 107 (88.4) | .015 |
| Acute MI | 104 (17.6) | 97 (20.6) | 7 (5.8) | <.001 |
| PCI | 87 (14.7) | 83 (17.7) | 4 (3.3) | <.001 |
| Peripheral artery disease | 32 (5.4) | 27 (5.7) | 5 (4.1) | .371 |
| Stroke | 38 (6.4) | 33 (7.0) | 5 (4.1) | .303 |
| Heart failure | 23 (3.9) | 19 (4.0) | 4 (3.3) | .584 |
| Previous treatment | ||||
| Antiplatelets | 222 (37.6) | 193 (41.1) | 29 (24.0) | <.001 |
| Beta-blockers | 160 (27.1) | 133 (28.3) | 27 (22.3) | .137 |
| ACE inhibitors | 276 (46.7) | 227 (48.3) | 49 (40.5) | .157 |
| Statins | 260 (44.0) | 215 (45.7) | 45 (37.2) | .048 |
ACE, angiotensin converting enzyme; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Data are expressed as No. (%) or mean±standard deviation.
Baseline characteristics on presentation and during admission
| Overall sample (n=591) | Patients with coronary obstructive lesions (n=470) | Patients with nonobstructive coronary arteries (n=121) | P | |
|---|---|---|---|---|
| Rest chest pain | 374 (63.3) | 285 (60.6) | 89 (73.6) | .004 |
| Single episode of chest pain | 324 (54.8) | 242 (51.5) | 82 (67.8) | .001 |
| Electrocardiogram | ||||
| ST descent | 136 (23.0) | 125 (26.6) | 11 (9.1) | < .001 |
| Negative T wave | 65 (11.0) | 53 (11.3) | 12 (9.9) | .460 |
| ↓ST or negative T | 201 (34.0) | 178 (37.9) | 23 (19.0) | <.001 |
| LBBB | 39 (6.6) | 23 (4.9) | 16 (13.2) | .002 |
| PM | 11 (1.9) | 4 (0.9) | 7 (5.8) | .001 |
| LBBB or PM | 49 (8.3) | 27 (5.7) | 22 (18.2) | <.001 |
| AF | 54 (9.1) | 36 (7.7) | 18 (14.9) | .021 |
| Systolic BP (mmHg)a | 144.6±30.2 | 144.5±30.7 | 144.8±28.3 | .89 |
| Diastolic BP (mmHg)a | 78.3±15.9 | 78.0±16.1 | 79.4±15.5 | .48 |
| Heart rate (bpm)a | 79.6±16.6 | 79.4±16.6 | 80.3±16.6 | .58 |
| KILLIP≥2 | 53 (9.0) | 48 (10.2) | 5 (4.1) | .048 |
| Hemoglobin (g/dL)a | 13.47±1.88 | 13.47±1.94 | 13.48±1.65 | .947 |
| White blood cells (x106/L)b | 8350 [6840-10 460] | 8500 [6907-10 502] | 7980 [6520-10 260] | .499 |
| Creatinine (mg/dL)b | 0.94 [0.8-1.21] | 0.95 [0.8-1.22] | 0.91 [0.8-1.18] | .148 |
| GFR (mL/min/m2)a | 63.30±21.54 | 63.1±20.8 | 64.01±23.88 | .677 |
| GFR<30 mL/min/m2 | 32 (5.4) | 20 (4.3) | 12 (9.9) | .034 |
| Troponin (ng/L) | ||||
| Initialb | 40.7 [19.7-116.8] | 44.7 [20.8-126.1] | 31.0 [16.8-63.0] | .001 |
| 2nd determinationb | 82.2 [31.7-233.6] | 92.5 [36.0-276.0] | 44.48 [20.4-118.9] | .001 |
| Peakb | 83.0 [32.4-238.3] | 96.5 [37.3-283.6] | 44.99 [22.3-125.3] | .001 |
| ΔTn (n=472)b | 19.45 [0.8-70.3] | 28.4 [1.7-84.12] | 7.35 [-0.7-33.7] | .078 |
| ΔTn<20 ng/L | 243 (50.4) | 165 (45.1) | 71 (67.2) | <.001 |
| ΔTn relativeb | 0.33 [0.02-1.5] | 0.36 [0.05-1.6] | 0.22 [-0.02-0.12] | .380 |
| ΔTn relative<20% | 193 (40.0) | 138 (37.7) | 51 (47.0) | .064 |
| LVEFa | 58.08±10.1 | 57.5±10.0 | 60.14±9.82 | .008 |
| LVEF>55% | 433 (73.3) | 334 (71.1) | 99 (81.8) | .008 |
| Left ventricular hypertrophy | 247 (41.8) | 196 (41.7) | 51 (42.1) | .943 |
| Wall motion abnormality | 208 (35.2) | 182 (38.7) | 26 (21.5) | < .001 |
| Number of diseased vessels | ||||
| 0 | 121 (20.5) | -- | ||
| 1 | 215 (36.4) | 215 (45.7) | ||
| 2 | 161 (27.2) | 161 (34.3) | ||
| 3 | 94 (15.9) | 94 (20) | ||
| Left main | 36 (6.1) | 36 (7.7) | ||
AF, atrial fibrillation; BP, blood pressure; GFR, glomerular filtration rate; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; PM, pacemaker: Tn, troponin.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Revascularization and treatment at discharge
| Overall sample (n=591) | Patients with coronary obstructive lesions (n=470) | Patients with nonobstructive coronary arteries (n=121) | P | |
|---|---|---|---|---|
| PCI | 313 (53) | 313 (66.6) | -- | |
| CABG | 58 (9.8) | 58 (12.3) | -- | |
| Aspirin | 541 (91.5) | 452 (96.2) | 89 (73.6) | < .001 |
| DAPT | 423 (71.6) | 388 (83.3) | 35 (28.9) | < .001 |
| Clopidogrel | 414 (70.1) | 376 (80.0) | 38 (31.4) | < .001 |
| Ticagrelor | 14 (2.4) | 14 (3.0) | -- | .048 |
| Prasugrel | 9 (1.5) | 9 (1.9) | -- | .219 |
| Statin | 535 (90.5) | 439 (94.2) | 96 (79.3) | < .001 |
| Betablocker | 509 (86.1) | 412 (88.4) | 97 (80.2) | .001 |
| ACE inhibitor | 470 (79.5) | 383 (82.2) | 87 (71.9) | .004 |
| Anticoagulation | 58 (9.8) | 41 (8.8) | 17 (14.0) | .020 |
ACE, angiotensin converting enzyme; CABG, coronary artery bypass grafting; DAPT: dual antiplatelet therapy; PCI, percutaneous coronary intervention.
Data are expressed as No. (%).
MINOCA was further classified according to angiographic findings as normal coronary arteries (ie, smooth coronary arteries [CA]), mild irregularities (< 30% stenosis), and moderate coronary atherosclerosis (30%-49% stenosis).15
The primary endpoint was a composite of mortality, nonfatal myocardial infarction, and revascularization (MACE) at a median follow-up of 5.1 years. Secondary endpoints were the individual components of the primary endpoint. Information at follow-up was collected in the outpatient department or by reviewing the electronic medical record.
Statistical analysisContinuous variables are presented as mean±standard deviation or as the median and interquartile range, while categorical variables are expressed as absolute values and percentages.
For the primary endpoint, univariate analysis was performed considering the clinical variables presented in table 1, table 2, and table 3. Then, all significantly associated (P<.05) variables were included in a multivariate Cox regression analysis using the backward stepwise regression method. After this, the resulting model was verified with forward stepwise (Wald) method. Results are expressed by the hazard ratio (HR) of each variable with a 95% confidence interval (95%CI) and statistical significance (P), and c-statistic of the models. Afterwards, the same method was applied to the secondary endpoints. In the case of reinfarction and revascularization events, an analysis was also performed taking death as a competing event using the Fine and Gray method to modify the Cox proportional hazards model.
A sensitivity analysis was performed in the MINOCA patient subgroup. Following the same methodology as in the previous paragraph, univariate and multivariate analyses were carried out using Cox regression to identify variables associated with the event, expressing their statistical significance and the magnitude of the effect. Patients were categorized according to their angiographic findings as smooth CA, vessel wall irregularities, and nonsignificant coronary stenosis.
For sample size calculation, a MINOCA prevalence of 20% was estimated.20 It was proposed to reach at least 100 MINOCA, so we planned to recruit at least 500 patients. Follow-up lasted a minimum of 5 years to assess the long-term prognosis of these patients and to achieve a significant number of events that allowed for multivariate adjustment.
ResultsDuring the study period, 603 patients were admitted with NSTEMI and underwent coronary angiography, and 133 of them showed nonobstructive CA. Intracoronary imaging was performed in 3 patients and fractional flow reserve assessment in 2 patients in the nonobstructive CA group, whose findings ruled out ruptured coronary plaques or significant functional coronary stenosis. After complete diagnostic work-up, including cardiac magnetic resonance in 40.6% of patients with nonobstructive CA, 12 patients were excluded due to an alternative diagnosis: 5 myocarditis, 1 tako-tsubo, 4 hypertrophic cardiomyopathy and 2 cases of extracardiac causes of myocardial injury. shows the study flowchart.
A total of 591 patients were included in the analysis, 68% were male and the mean age was 69 years; 121 patients (20.5%) showed no obstructive lesions on coronary angiography; among these, 61.9% were angiographically normal, 22.3% had mild irregularities (< 30% stenosis), and 15.7% moderate coronary atherosclerosis (30%-49% stenosis). Table 1, table 2, and table 3 show baseline, hospital admission, and management characteristics of the overall sample and the subgroups of obstructive and nonobstructive CA. In brief, patients with nonobstructive CA were more frequently female, with a lower prevalence of diabetes, smoking, previous MI, and percutaneous coronary intervention, and less treatment with antiplatelet therapy and statins. Regarding episode characteristics, MINOCA patients more frequently had chest pain at rest and a single episode, fewer ST segment descent but a higher prevalence of left bundle branch block, pacemaker rhythm, and atrial fibrillation (AF). All troponin levels were lower in the MINOCA group, and a higher percentage of patients had a delta Tn<20 ng/L, without significant differences in relative changes. All recorded treatments were less frequently used in the MINOCA group, except for a higher use of anticoagulant therapy.
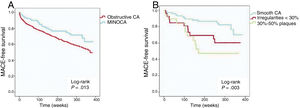
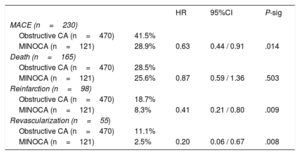
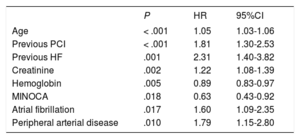
Prognostic impact of MINOCAAt a median follow-up of 5.1 years (maximum of 91 months), MACE occurred in 38.9% (230) of the patients; individual endpoint rates were 27.9% (n=165) for death, 16.6% (n=98) for reinfarction, and 9.3% (n=55) for revascularization. MINOCA was significantly associated with a lower occurrence of MACE (P=.014; HR, 0.63; 95%CI, 0.44-0.91) (table 4, figure 1A). Then, a multivariate model for MACE was built including MINOCA diagnosis and all variables significantly associated in the univariate analysis. MINOCA remained in the model as an independent predictor of MACE (P=.018; HR, 0.63; 95%CI, 0.43-0.92) together with age, previous percutaneous coronary intervention, a history of heart failure, creatinine and hemoglobin levels, AF, and peripheral artery disease (table 5). The AUC of the model was 0.783.
Univariate Cox regression. Impact of MINOCA and angiographic subtypes on the presence of MACE and its individual components (death, reinfarction, and revascularization) during follow-up (n=591).
| HR | 95%CI | P-sig | ||
|---|---|---|---|---|
| MACE (n=230) | ||||
| Obstructive CA (n=470) | 41.5% | |||
| MINOCA (n=121) | 28.9% | 0.63 | 0.44 / 0.91 | .014 |
| Death (n=165) | ||||
| Obstructive CA (n=470) | 28.5% | |||
| MINOCA (n=121) | 25.6% | 0.87 | 0.59 / 1.36 | .503 |
| Reinfarction (n=98) | ||||
| Obstructive CA (n=470) | 18.7% | |||
| MINOCA (n=121) | 8.3% | 0.41 | 0.21 / 0.80 | .009 |
| Revascularization (n=55) | ||||
| Obstructive CA (n=470) | 11.1% | |||
| MINOCA (n=121) | 2.5% | 0.20 | 0.06 / 0.67 | .008 |
95%CI, 95% confidence interval; CA, coronary arteries; HR, hazard ratio; MACE, major cardiovascular events (death, reinfarction, and revascularization); MINOCA, myocardial infarction with nonobstructive coronary arteries.
Data are expressed as percentage of events.
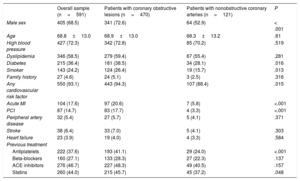
Kaplan-Meier curves. A: MACE at follow-up according to the diagnosis of MINOCA or obstructive coronary arteries (CA). B: MACE in the MINOCA group according to angiographic subtypes of MINOCA. MACE, major cardiovascular events (death, reinfarction, and revascularization); CA, coronary arteries; MINOCA, myocardial infarction with nonobstructive coronary arteries.
Cox regression. Predictive model of the presence of MACE during follow-up (n=591).
| P | HR | 95%CI | |
|---|---|---|---|
| Age | < .001 | 1.05 | 1.03-1.06 |
| Previous PCI | < .001 | 1.81 | 1.30-2.53 |
| Previous HF | .001 | 2.31 | 1.40-3.82 |
| Creatinine | .002 | 1.22 | 1.08-1.39 |
| Hemoglobin | .005 | 0.89 | 0.83-0.97 |
| MINOCA | .018 | 0.63 | 0.43-0.92 |
| Atrial fibrillation | .017 | 1.60 | 1.09-2.35 |
| Peripheral arterial disease | .010 | 1.79 | 1.15-2.80 |
95%CI, 95% confidence interval; HF, heart failure; HR, hazard ratio; MACE, major cardiovascular events (death, reinfarction, and revascularization); MINOCA, myocardial infarction with nonobstructive coronary arteries; PCI, percutaneous coronary interventions.
Univariate analysis of the separate components of the main endpoint showed that MINOCA was significantly associated with a lower rate of MI (P=.009; HR, 0.41; 95%CI, 0.21-0.80) and revascularization (P=.008; HR, 0.20; 95%CI, 0.06-0.67) at follow-up (table 4). This association was maintained in the multivariate analysis. However, the statistical association between MINOCA and mortality was not significant.
Finally, an analysis was carried out in the MINOCA patients (n=121). MACE was observed in 28.1% of the patients. Kaplan-Meier curves showed that, among the different angiographic subtypes, the presence of smooth CA was significantly associated with a lower occurrence of MACE (log-rank P=.003) (figure 1B). Indeed, in the univariate Cox regression analysis, both vessel wall irregularities (P=.03; HR, 2.45; 95%CI, 1.09-5.53) and nonsignificant stenosis (P=.002; HR, 3.64; 95%CI, 1.61-8.23) had a significantly higher risk of MACE at follow-up compared with smooth CA (table 6). Multivariate analysis showed that smooth CA (P=.015; HR, 0.40; 95%CI, 0.19-0.84), aspirin treatment at discharge (P=.019; HR,0.42; 95%CI, 0.21-0.87) and age (P=.046; HR,1.04; 95%CI, 1.01-1.09) were the only variables independently associated with MACE.
Univariate Cox regression. Impact of angiographic subtypes of MINOCA in the presence of MACE within the MINOCA group during follow-up (n=121).
| MACE (n=34) | HR | 95%CI | P-sig | |
|---|---|---|---|---|
| Smooth CA (n=75) | 18.7 | reference | ||
| Mild irregularities<30% (n=27) | 37.0 | 2.45 | 1.09-5.53 | .03 |
| Plaques 30%-50% (n=19) | 52.6 | 3.64 | 1.61-8.23 | .002 |
95%CI, 95% confidence interval; CA, coronary arteries; HR, hazard ratio; MACE, major cardiovascular events (death, reinfarction and revascularization); MINOCA, myocardial infarction with nonobstructive coronary arteries.
Data are expressed as percentage of events.
The results of our study show a 20% prevalence of nonobstructive CA in an unselected series of patients with NSTEMI. Unlike other studies in the field, we focused on long-term follow-up, as well as on the prognostic role of the angiographic subtypes. MINOCA diagnosis was associated with fewer MACE (combined death, reinfarction, and revascularization) at 5-year follow-up, mainly driven by the lower number of reinfarctions and revascularizations. Moreover, the subgroup of smooth CA had the best prognosis within MINOCA patients, while those with plaques of 30%-49% showed a higher rate of MACE.
Epidemiology of MINOCAThe prevalence of nonobstructive CA in our series of NSTEMI patients was 20.5%. This is a high value compared with the data in the literature. Pasupathy et al. found a prevalence of MINOCA of 6% in a meta-analysis.10 However, there is significant variability (between 5% and 25%) in published series.1–11 This variability can be attributed to various factors: differences in inclusion criteria (full spectrum of MI vs only STEMI or NSTEMI), lack of homogeneity in MINOCA diagnostic, recent widespread use of highly sensitive Tn, inter- and intraobserver variability in visual interpretation of angiography, variable percentage of coronary angiography indication in the context of type 2 MI, use of complementary tests to establish a specific diagnosis (cardiac magnetic resonance, imaging or intracoronary physiology techniques).3,14,15,21–26 In short, heterogeneity in the data reflects incomplete knowledge of this entity. Therefore, it is especially important in clinical practice to use a strict definition of MINOCA (following the standardized definition in the ESC and AHA recommendations) and to carry out an exhaustive study aimed at identifying the underlying cause in as many patients as possible.14,15 Our series may also be influenced by these limitations, especially by a low use of complementary techniques. However, current clinical practice in our setting is reflected by the use of highly sensitive Tn, the follow-up of current clinical guidelines and the use of the recommended criteria for the diagnosis of MINOCA in recent consensus.
MINOCA prognosisBroadly, the literature addressing this topic is based on observational studies that are heterogeneous in terms of inclusion and exclusion criteria, evaluation variables (usually total mortality) and follow-up (most provide data at 1 year). We decided to adhere to the most recent recommendations for MINOCA diagnosis, assess the occurrence of major cardiovascular events and plan a long-term follow-up, since the literature data in this regard are scarce. Specifically, patients with a nonischemic discharge diagnosis (ie, cardiomyopathy, nonischemic myocardial injury or noncardiological disease) were excluded, because they constitute independent entities with differentiated management and prognosis.
Overall, our results indicate that the MINOCA in NSTEMI patients is independently associated with a significantly lower number of major cardiovascular events (death, reinfarction, and new revascularization) in long-term follow-up compared with patients with obstructive CA. Published data on this topic are controversial. Most studies show a better mortality prognosis of MINOCA.1,4,9,10,12 In a meta-analysis, Pasupathy et al. found 4.7% mortality at 12 months (similar to the 4.5% in our study), 6 of the included studies compared the event against patients with obstructive CA, finding a significantly lower mortality in the MINOCA group (3.5% vs 6.7%; odds ratio, 0.59, 0.41-0.83; P=.003)10. The study with the longest follow-up found a lower rate of mortality and reinfarction at 2 years in these patients.4 On the other hand, some studies found no better prognosis of MINOCA. A propensity matched analysis of the ACUITY clinical trial found higher mortality at 12 months in patients with NSTEMI and MINOCA compared with obstructive CA (5.2% vs 1.6%), mainly derived from noncardiovascular mortality; however, the latter group showed a higher rate of reinfarction and revascularization.13 Andersson et al.2 found no overall mortality difference in STEMI, although MINOCA patients had lower cardiovascular mortality. One-year mortality has also been reported as similar in young patients and comparing MINOCA to 1- or 2-vessel significant disease.8,27
Angiographic subtypes of MINOCAThe recommended angiographic definition of nonobstructive CA (less than 50% stenosis) includes a variable degree of coronary artery disease. For this reason, consensus documents on MINOCA recommend an angiographic classification that distinguishes coronary events with a total absence of atherosclerosis from those with nonobstructive atherosclerosis since they can translate different entities and have diagnostic and prognostic implications.14,15 However, to date the evidence on the impact of these angiographic subtypes of MINOCA on prognosis is very limited.
We found that smooth CA (ie, no evidence of atherosclerosis on angiography) were significantly associated with a lower number of MACE compared with the other angiographic subtypes (ie, mild irregularities<30% and plaques 30%-50%). These findings support the hypothesis that angiographic subgroups are independent entities within MINOCA, possibly with different pathophysiological mechanisms and with diagnostic-therapeutic implications. More studies on this topic are needed to confirm this hypothesis. In a nonsystematic review, Di Fiore et al.28 collected a series of MINOCA studies that offered differential data on smooth CA and nonsignificant CA disease; it was suggested that total mortality is lower in patients with smooth CA, although there was no analysis providing statistical significance. Bugiardini et al.16 performed a meta-analysis of patients with NSTEMI from 3 clinical trials in the TIMI group and found that patients with smooth CA had fewer events at follow-up than patients with nonsignificant atherosclerosis, but only revascularization was individually significant. On the other hand, Bainey et al.17 found no significant differences in mortality or reinfarction rate at 1 year depending on the angiographic type, and Andersson et al.2 observed a higher mortality (mainly driven by noncardiovascular mortality) in the smooth CA group vs obstructive CA in STEMI patients.
The worse prognosis of MINOCA with nonsignificant atherosclerosis could be explained by the fact that coronary stenosis or its physiological repercussion have been undervalued in coronary angiography, because medical treatment related to secondary prevention is less implemented in this type of patient, or that these findings are a risk marker translating a subclinical atherosclerotic disease but related to events in the follow-up. In either of these cases, this situation would carry a risk of atherothrombotic events during follow-up. Several findings support these hypotheses. First, coronary angiography shows high interobserver variability in estimating lesion severity.23 Second, the evidence of unstable plaques not visible on coronary angiography: small case series have found that about a third of MINOCA patients have ruptured or eroded atherosclerotic plaques on IVUS (intracoronary ultrasound) analysis, and this may be even higher if evaluated by optical coherence tomography (OCT).29–31 Third, inaccurate correlation between the degree of stenosis and the functional repercussion of a coronary lesion: a consensus document suggests the possible role of intracoronary functional assessment in selected cases, although not supported by evidence.15 Fourth, evidence of atherosclerosis progression in patients with MINOCA: in an analysis of 9092 patients with MINOCA from the Swedish registry SWEDEHEART, obstructive CA disease was found in nearly half of patients with recurrent MI who underwent coronary angiography at follow-up.32 Finally, the benefit of medical treatment in patients with MINOCA: although scarce, some observational data suggest the benefit of medications with an established role in coronary heart disease in MINOCA, such as angiotensin converting-enzyme inhibitors and beta-blockers.33 All these findings highlight both the need for further investigation on MINOCA diagnosis and management and the importance of complete etiological diagnosis in each of these patients.
In the multivariate analysis performed in our study, smooth CA confirmed its independent association with lower MACE, together with antiplatelet therapy with aspirin and age. The small number of patients included for this purpose limits the conclusions. However, these results reinforce the possible role of the angiographic subtype of smooth CA in the prognosis of these patients. Nordenskjöld et al.34 evaluated the independent predictors of MACE in MINOCA patients from the SWEDEHEART registry, and they found a clinical high-risk profile (age, hypertension, smoking, stroke, peripheral artery disease, low LVEF, among other factors) related to events, but it did not include characteristics of the MINOCA episode.
LimitationsVarious limitations can be recognized in this work. First, this is a single-center study and the results may be influenced by local peculiarities in patient management. The number of patients may limit conclusions, especially regarding subgroup analysis, which should only be considered as hypothesis generating. Angiographic classification of nonobstructive CA was performed using subjective visual estimation, which may limit reproducibility. Finally, a systematic etiological study was not carried out (ie, intracoronary imaging, microvascular function assessment, cardiac magnetic resonance, etc) and therefore no conclusions can be drawn about the influence of the underlying pathophysiological processes on prognosis and their possible relationship with angiographic subtypes, and the lack of systematic etiological study may have led to overestimation of the MINOCA rate in the cohort.
ConclusionsIn an unselected cohort of patients with NSTEMI who underwent coronary angiography, MINOCA diagnosis was associated with a lower rate of major cardiovascular events (death, reinfarction, and revascularization) in long-term follow-up compared with obstructive CA. This was primarily driven by fewer reinfarctions and revascularizations. Among the angiographic subtypes of MINOCA, only the presence of smooth CA was significantly and independently associated with a lower number of MACE and its components.
- -
MINOCA is a frequent entity, accounting for 5% to 25% of myocardial infarctions.
- -
Discordant data have been found on the prognosis of patients with MINOCA. While most studies have found a better prognosis than obstructive coronary lesions, others have reported similar or even worse prognosis. Moreover, long-term prognosis is less well documented.
- -
It has been recently recommended to further classify MINOCA according to angiographic subtypes
- -
MINOCA was associated with lower MACE at long-term follow-up (median 61.6 months) compared with obstructive CA, mainly driven by fewer reinfarctions and revascularizations with no significant difference in mortality.
- -
Smooth CA (no evidence of angiographic atherosclerosis) was independently associated with lower MACE.
- -
Within MINOCA patients, mild irregularities (< 30% stenosis) and moderate coronary atherosclerosis (30%-49%) showed a higher rate of MACE.
- -
The only independent prognostic factors within the MINOCA population were smooth CA, aspirin therapy, and age.
Spanish Ministry of Economy and Competitiveness through the Carlos III Health Institute: CIBER-CV 16/11/00420, Madrid, Spain.
Conflicts of interestNone declared.
Supplementary data associated with this article can be found in the online version, at